Abstract
17α-Ethinylestradiol (EE2), a synthetic and potent estrogen receptor agonist, is extensively metabolized in both intestine and liver and is largely excreted in bile and urine as the 3-O-sulfate (EE2-Sul) and 3-O-glucuronide. In the present study, EE2-Sul was evaluated as a substrate of various transporters known to be expressed in the kidney. Uptake studies were performed with human epithelial cells [human embryonic kidney (HEK)-293] that contained individually expressed organic cation transporter 2 (OCT2), organic anion transporter (OAT) forms 3 and 4, and multidrug and toxin extrusion 1 (MATE1). The transporter phenotyping studies were extended to include insect cell (Sf9) membrane vesicles that expressed multidrug resistance-associated protein 4 (MRP4) and Madin-Darby canine kidney cells that expressed OAT1. Based on the results obtained, we concluded that EE2-Sul serves as a substrate of OAT3 and OAT4, but not OCT2, OAT1, MATE1, and MRP4. First, EE2-Sul uptake was highly increased in OAT3/HEK-293 cells (versus mock/HEK-293 cells) and was inhibited by OAT3 inhibitors such as bromosulfophthalein (BSP), cimetidine, and probenecid. OAT3-mediated uptake also conformed to single-Km (Michaelis constant) kinetics (Km = 21.1 μM). Second, EE2-Sul uptake was also significantly higher in OAT4/HEK-293 cells and was inhibited by BSP, methotrexate, and probenecid. In contrast to OAT3, OAT4-dependent uptake was characterized by a two-Km model (Km1 = 1.6 μM; Km2 = 195 μM). Based on the results of this study, we hypothesize that EE2-Sul is taken up into renal proximal tubule cells by OAT3, and OAT4 plays a role in its secretion into the renal brush border lumen.
A synthetic and potent estrogen receptor agonist, 17α-ethinylestradiol (EE2) is a major estrogen component of oral contraceptive formulations (Zhang et al., 2007). Although EE2 is well absorbed, oral bioavailability is variable (e.g., 20–65%) because of extensive first-pass metabolism in both the intestine and liver. Such metabolism involves sulfotransferase-catalyzed 3-O-sulfation, UDP-glucuronosyltransferase-catalyzed 3-O-glucuronidation, and cytochrome P450-mediated 2-hydroxylation. Of the three pathways, sulfation dominates and the resulting metabolite (EE2-Sul) circulates at concentrations that are at least one order of magnitude greater than the parent EE2 (Back et al., 1980). The results of various pharmacokinetic and radiolabeled studies have demonstrated that EE2 undergoes enterohepatic recirculation, with the various metabolites recovered in bile (∼40% of dose) and urine (∼30% of the dose) (Maggs et al., 1983). The presence of both EE2-Sul and EE2-Glu in the urine is thought to reflect active transport in kidneys (Maggs et al., 1983).
In an accompanying manuscript (Han et al., 2010), EE2-Sul was shown to be a substrate of numerous liver-expressed transporters (OATP1B1, OATP2B1, NTCP, and BCRP). However, information related to the transporters involved in the renal excretion of EE2-Sul is lacking. In the human kidney, a variety of solute carrier (SLC) and ATP-binding cassette (ABC) transporters are expressed in proximal tubule cells and play a major role in the uptake and secretion of organic compounds (Lee and Kim, 2004; Robertson and Rankin, 2006). Namely, organic anion transporter (OAT)1 and OAT3 are SLCs expressed on the basolateral membrane, whereas OAT4, multidrug resistance-associated protein (MRP)2, MRP4, and BCRP are apical transporters known to actively transport organic anions. However, additional transporters, such as organic cation transporter (OCT)2 (e.g., prostaglandins) and MATE1 (e.g., estrone-3-sulfate, acyclovir, and ganciclovir), are also able to transport organic anions (Tanihara et al., 2007). Therefore, the purpose of the present study was to characterize the drug transporters that are responsible for renal excretion of EE2-Sul. Studies were performed with HEK-293 cells containing stably expressed OCT2, OAT3, OAT4, and MATE1, Madin-Darby canine kidney (MDCK) cells expressing OAT1, and insect cell (Sf9) membrane vesicles expressing MRP4. It is concluded that circulating EE2-Sul is taken up by OAT3 and effluxed by OAT4 at the brush-border membrane of human renal proximal tubule cells. The latter may function coordinately with BCRP, because EE2-Sul is also a BCRP substrate (Han et al., 2010). Therefore, at least three transporters may play a role in the renal elimination of EE2-Sul.
Methods and Materials
Materials.
[3H]EE2 (60 Ci/mmol) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO). [3H]Estrone-3-sulfate ammonium salt (2.12 TBq/mmol), [3H]estradiol-17β-d-glucuronide (1.73 TBq/mmol), [3H]PAH (161 GBq/mmol), and [3H]MPP (3.16 TBq/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Unlabeled EE2-Sul was obtained from Steraloids (Newport, RI). Human liver cytosol was purchased from BD Biosciences (Palo Alto, CA). All other reagents were commercial products of reagent grade. HEK-293 cells that contained the Flp recombination target (FRT) recombination site were purchased from Invitrogen (Carlsbad, CA). MDCK cells expressing OAT1 were kindly provided by Dr. John Pritchard (National Institute of Environmental Health Sciences, Research Triangle Park, NC). Membrane vesicles prepared from Sf9 cells expressing human MRP4 were purchased from Genomembrane, Inc. (Yokohama, Japan).
Synthesis of [3H]EE2-Sul.
Biosynthesis of [3H]EE2-Sul from [3H]EE2 was based on the methods described previously (Li et al., 1999 and Schrag et al., 2004). [3H]EE2 (250 μCi) was transferred in ethanol into a test tube with a screw cap. The sample was gently evaporated under a stream of nitrogen to near dryness. A stir bar was added to the test tube followed by addition of 0.4 mg of PAPS [in 0.2 ml of phosphate buffer (0.1 M), pH 7.0], 100 μl (2 mg) of pooled human liver cytosol (BD Biosciences), and 0.7 ml of phosphate buffer (0.1 M), pH 7.0. The reaction was started at 37°C and its progress was monitored by radio-high-performance liquid chromatography (HPLC). A second batch of PAPS [2 mg in 0.5 ml of phosphate buffer (0.1 M), pH 7.0] was added to the reaction mixture after 2 h. The reaction was stopped after 4 h, at which point more than 80% of the radiolabeled EE2 present was consumed. Radio-HPLC analysis indicated that the EE2-Sul product represented 54.3% of the total radioactivity in the incubate. The reaction was quenched by addition of 5 ml of ethanol, and the protein precipitate was filtered through a Pasteur pipette filled with glass wool and filter paper. The clear reaction solution was concentrated by rotary evaporation, and the product was purified by HPLC on a Phenomenex Luna(2) 10 × 250 mm column (Phenomenex, Torrance, CA) eluted with solvent A (water containing 0.1% trifluoroacetic acid) and solvent B (acetonitrile). The mobile phase was programmed as follows: 30% solvent B and 70% solvent A (0–30 min), 30 to 50% solvent B and 70 to 50% solvent A (30–40 min), and 50 to 100% solvent B, with 50 to 0% solvent A (40–45 min), at a flow rate of 4.5 ml/min (UV detector wavelength set at 215 nm). Fractions that contained the pure product (32–34 min) were pooled and rotary evaporated. The final product was reconstituted in 90% ethanol to give 190 μCi of [3H]EE2-Sul (76% yield; radiochemical purity >99%). The specific activity was 49.8 Ci/mmol based on the abundance of radiolabels measured by liquid chromatography-mass spectrometry.
Stable Transfection of OCT2, OAT3, OAT4, and MATE1 in HEK-293 Cells.
HEK-293 cells were routinely grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum in a humidified incubator at 37°C and 5% CO2. The stably transfected HEK-293 cell lines were established by using the Flp-In expression system (Invitrogen) according to the manufacturer's protocol. In brief, in separate reactions, the cDNAs, including the open reading frames of OCT2, OAT3, OAT4, and MATE1, were subcloned into the Flp-In expression vector pcDNA5/FRT, which contained a FRT site linked to the hygromycin resistance gene. The constructs pcDNA5/FRT-OCT2, pcDNA5/FRT-OAT3, pcDNA5/FRT-OAT4, and pcDNA5/FRT-MATE1 were then cotransfected with the Flp recombinase expression vector pOG44 into Flp-In HEK-293 cells. Cells stably expressing the transporters were selected in hygromycin (100 μg/ml) according to the manufacturer's protocol. The cells were grown in flasks cultured in Dulbecco's modified minimum essential medium (Invitrogen) supplemented with 10% fetal bovine serum and hygromycin (100 μg/ml). Cultures were maintained in a humidified atmosphere containing 5% CO2 at 37°C. Cells were split in a 1:5 ratio every 3 to 4 days.
Real-Time Polymerase Chain Reaction Assay.
Total RNA was isolated from OCT2/HEK-293 cells, OAT3/HEK-293 cells, OAT4/HEK-293 cells, MATE1/HEK-293 cells, and mock/HEK-293 cells using RNeasy Mini Kit (QIAGEN, Valencia, CA). The total RNA obtained was quantified using the Agilent RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA) and detected with Agilent 2100 Bioanalyzer (Agilent Technologies).
After the RNA isolation, a two-step fast real-time polymerase chain reaction (PCR) assay was performed, using a 7900 Fast PCR System instrument (Applied Biosystems, Foster City, CA), according to the manufacturer's relative quantification (RQ) protocol. For the reverse transcription step, cDNA was reverse-transcribed from 2 μg of total RNA using random primers from a high-capacity cDNA archive kit (Applied Biosystems). In the PCR step, a master mixture solution was prepared using a TaqMan Fast Universal PCR Master Mix without AmpErase UNG (Applied Biosystems). All TaqMan assay primers (OCT2, Hs 00533907_m1; OAT3, Hs 00188599_m1; OAT4, Hs 00945829_m1; MATE1, Hs 00217320_m1; and β-actin, Hs 9999993 m1) and probes were ordered from Applied Biosystems.
The thermal cycling parameters were 20 s at 95°C (denaturing), 40 cycles in 3 s at 95°C (melting), and 30 s at 60°C (annealing and extending). Human β-actin gene was used as an endogenous control. Quantitative real-time PCR of the target mRNA and actin beta was run in the same plate but in different wells. The comparative cycle-threshold method (ΔΔCT method) was used to determine the RQ for each gene of interest. RQ values for each of the genes were then expressed as fold of control (mock/HEK-293 cells).
Transport Studies with Transporter-Expressing HEK-293 and MDCK Cells.
For uptake measurements, the stably transfected HEK-293 (OCT2, OAT3, OAT4, and MATE1) and MDCK (OAT1 only) cells and mock control cells were seeded on BioCoat poly-d-lysine-coated 24-well plates (BD Biosciences) at a density of 2.5 × 105 cells per well. After 2 days, the cells were washed twice with 2 ml of Hanks' balanced salt solution [(HBSS) 137 mM NaC1, 5.36 mM KCl, 0.20 mM MgSO4, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 4.17 mM NaHCO3, 1.26 mM CaC12, and 5.6 mM glucose] and incubated with radiolabeled substrate dissolved in HBSS supplemented with 10 mM HEPES (pH 7.4) at 37°C. MPP (1 μM, OCT2 and MATE1), PAH (1 μM, OAT1), and estrone-3-sulfate (1 μM, OAT3 and OAT4) were used as substrates for the different transporters. In addition, the inhibitors of each transporter were selected as follows: imipramine (200 μM, OCT2), bromosulfophthalein (BSP) (5 or 50 μM, OAT1, OAT3 and OAT4), pyrimethamine (1 μM, MATE1), probenecid (10, 50, or 100 μM, OAT3 and OAT4), cimetidine (10 and 100 μM, OAT3), and methotrexate (100 and 500 μM, OAT4). The incubation of the cells with radiolabeled compounds was stopped at the designated time by removing the medium and washing the cells twice with 2 ml of ice-cold HBSS solution. Kinetic assessment of EE2-Sul uptake involved incubations (3 min) of a constant amount of radiolabel, with varied amounts of unlabeled substrate. In all cases, the cells were lysed with 0.3 ml of lysis buffer (containing 0.1 N NaOH and 0.1% SDS), and the radioactivity was determined by liquid scintillation counting. Total cellular protein was measured using the BCA protein assay kit supplied by Pierce (Rockford, IL).
Transport Studies with MRP4-Expressing Membrane Vesicles.
The transport studies were performed using a rapid filtration technique according to the manufacturer's protocol with a minor modification (Genomembrane, Inc., Yokohama, Japan). In brief, 30 μl of transport medium [50 mM MOPS-Tris (pH 7.0), 70 mM KCl, 7.5 mM MgCl2, and 2 mM glutathione], which contained membrane vesicles (50 μg of protein) and test compounds, was preincubated at 37°C for 5 min and then rapidly mixed with 20 μl of the reaction mixture containing 4 mM ATP or AMP. At the appropriate time, the transport was stopped by addition of cold wash buffer [200 μl, 40 mM MOPS-Tris (pH 7.0), 70 mM KCl]. The incubation mix was then rapidly transferred to a PerkinElmer Unifilter GF/B plate followed by five more 250-μl washes using a FilterMate Harvester (PerkinElmer Life and Analytical Sciences). Radioactivity in each well was determined using a PerkinElmer Top Count NXT Microplate Scintillation and Luminescence Counter. ATP-dependent transport was calculated by subtracting the values obtained in the presence of AMP from those in the presence of ATP.
Estimation of Kinetic Parameters.
To estimate kinetic parameters for saturable transport, the uptake rate (V) was fitted to the following equations by means of nonlinear least-squares regression analysis using WinNonlin (Scientific Consulting Inc., Cary, NC).
The kinetic parameters describing the uptake of EE2-Sul were obtained by using the following equations: in the case of a single saturable component (OAT3), V = (Vmax · C)/(Km+ C); and for a system consisting of two saturable components (OAT4), V = (Vmax1 · C)/(Km1+ C) + (Vmax2 · C)/(Km2 + C), where V and C are the uptake rate and concentration of substrate, respectively, and Km and Vmax represent the half saturation concentration (Michaelis constant) and the maximum transport rate, respectively. OAT3 and OAT4 uptake activity in HEK-293 cells was determined after subtracting the uptake by mock-transfected cells from total uptake by the OAT3- or OAT4-expressing cells.
All data are expressed as the mean ± S.D., and statistical analysis was performed by a two-way analysis of variance followed by a post hoc Dunnett's test. The criterion of significance was p < 0.05 or 0.01.
Results
Uptake of EE2-Sul into OCT2-Expressing HEK-293 Cells.
Before conducting transport studies, stably transfected OCT2-expressing HEK-293 cells were subjected to real-time PCR analysis, and the results revealed significant overexpression (∼400,000-fold) of OCT2 (versus mock cells) (data not shown). Furthermore, differential uptake of MPP into OCT2/HEK-293 cells (versus mock/HEK-293 cells) was evident (Table 1). The uptake of MPP was also inhibited (>90%) by imipramine (200 μM), a known OCT inhibitor (Urakami et al., 2001). In contrast to MPP, differential uptake of EE2-Sul into OCT2/HEK-293 cells was not observed and imipramine had no effect. Under the conditions of the study, therefore, EE2-Sul did not behave as an OCT2 substrate (Table 1).
Assessment of EE2-Sul as an OCT2, OAT1, and MATE1 substrate
The details of measurement are described under Materials and Methods.
Uptake of EE2-Sul into OAT1-, OAT3-, and OAT4-Expressing Cells.
As shown previously (Aslamkhan et al., 2003), it was evident that PAH uptake into OAT1/MDCK cells was significantly higher (15.9 versus 0.77 pmol/mg/3 min) than mock/MDCK cells (Table 1). The OAT1-mediated uptake was inhibited by an OAT1 inhibitor (BSP, 50 μM), suggesting that OAT1/MDCK cells were functionally active. In parallel, when EE2-Sul uptake was measured, there was no evidence for differential uptake into OAT1/MDCK cells (Table 1). Therefore, as with OCT2, EE2-Sul did not serve as a substrate of OAT1.
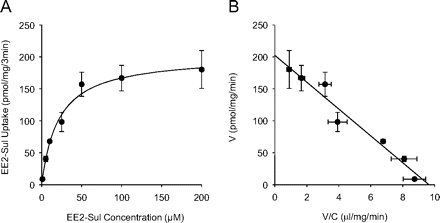
Both OAT3/HEK-293 cells and mock/HEK-293 cells were subjected to real-time PCR analysis, and the overexpression of OAT3 in the former (243,000-fold) was confirmed (data not shown). The high expression of OAT3 was reflected in the differential uptake of estrone-3-sulfate, which was inhibited (>90%) by BSP (Fig. 1A). When the uptake by mock and OAT3/HEK-293 cells was compared, significantly high uptake of EE2-Sul was apparent with OAT3/HEK-293 cells (Fig. 1B). The uptake was reduced with OAT3 inhibitors (BSP, cimetidine, probenecid) in a concentration-dependent manner, but no change was observed with TEA (noninhibitor) (Fig. 1C). These results suggest EE2-Sul is an OAT3 substrate. As shown in Fig. 2A, EE2-Sul (10 nM–200 μM) uptake increased nonlinearly with increasing concentration. The data were plotted using the Eadie-Hofstee equation to derive estimates of the kinetic parameters required for the nonlinear regression analysis and to assess the number of transport systems involved. As shown in Fig. 2B, the Eadie-Hofstee plot (V versus V/C) of EE2-Sul transport presented one linear component, which was described by single-Km (Km = 21.1 ± 2.7 μM; Vmax 67.5 ± 3.5 pmol/mg/min).
Uptake of estrone-3-sulfate (A) and EE2-Sul (B) into HEK-293 cells containing stably expressed OAT3, and inhibition of EE2-Sul uptake by inhibitors (C). A, uptake of estrone-3-sulfate (0.1 μM) into mock and OAT3/HEK-293 cells was determined after 3 min of incubation in the absence (■) and presence (□) of BSP (50 μM). B, uptake of EE2-Sul into mock (○) and OAT3/HEK-293 cells (●) was determined. C, cellular uptake of EE2-Sul (0.05 μM) was determined in the absence and presence of transporter inhibitors and reported as a percentage of EE2-Sul-only control. Data are shown as the mean ± S.D. (n = 3 determinations). **, p < 0.01 versus control.
Concentration dependence (A) and Eadie-Hofstee plot (B) describing the uptake of EE2-Sul into OAT3/HEK-293 cells. A, EE2-Sul (10 nM–200 μM) uptake into mock and OAT3/HEK-293 cells was measured, and OAT3-mediated uptake was obtained by subtracting the uptake into mock/HEK-293 cells from OAT3/HEK-293 cells. B, the uptake kinetics was described as an Eadie-Hofstee plot. Each point with bar represents the mean ± S.D. (n = 3 determinations).
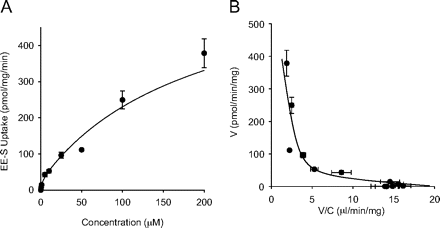
As with OAT3, real-time PCR results showed high expression of OAT4 mRNA in OAT4/HEK-293 cells (217,000-fold) (data not shown), and differential (BSP-inhibited) uptake of estrone-3-sulfate and EE2-Sul was also observed (Fig. 3A). EE2-Sul showed preferential uptake into OAT4/HEK-293 cells over mock/HEK-293 cells (Fig. 3B). The uptake by OAT4/HEK-293 cells was decreased in the presence of OAT4 inhibitors, BSP, methotrexate, and probenecid, but not TEA (noninhibitor) (Fig. 3C). OAT4-mediated uptake was saturable, as EE2-Sul concentration increased (Fig. 4A). To confirm the type of EE2-Sul transport, the OAT4-dependent uptake of EE2-Sul (10 nM–200 μM) into HEK-293 cells was analyzed using the Eadie-Hofstee plot and shown in Fig. 4B. Unlike OAT3, the plot characterized two obviously different transport systems: a high-affinity, low-velocity system (Km1 = 1.6 ± 1.8 μM, Vmax1 = 27.5 ± 21.42 pmol/min/mg) and a low-affinity, high-velocity system (Km2 = 195 ± 138 μM, and Vmax2 = 599 ± 225 pmol/min/mg). Therefore, EE2-Sul behaved as an OAT3 and OAT4 substrate.
Uptake of estrone-3-sulfate (A) and EE2-Sul (B) into HEK-293 cells containing stably expressed OAT4, and inhibition of EE2-Sul uptake by inhibitors (C). A, uptake of [3H]estrone-3-sulfate (0.1 μM) into mock and OAT1/MDCK cells was determined after 3 min of incubation in the absence (■) and presence (□) of BSP (50 μM). B, uptake of EE2-Sul into mock (○) and OAT4/HEK-293 cells (●) was determined. C, cellular uptake of EE2-Sul (0.05 μM) was determined in the absence and presence of transporter inhibitors and reported as a percentage of EE2-Sul-only control. Data are shown as the mean ± S.D. (n = 3 determinations). **, p < 0.01 versus control.
Concentration dependence (A) and Eadie-Hofstee plot (B) describing the uptake of EE2-Sul into OAT4/HEK-293 cells. A, [3H]EE2-Sul (10 nM–200 μM) uptake into mock and OAT4/HEK-293 cells was measured, and OAT4-mediated uptake was obtained by subtracting the uptake into mock/HEK-293 cells from OAT4/HEK-293 cells. B, the uptake kinetics was described as an Eadie-Hofstee plot. Each point with bar represents the mean ± S.D. (n = 3 determinations).
Uptake of EE2-Sul into MATE1-Expressing HEK-293 Cells.
Overexpression of MATE1 mRNA in MATE1/HEK-293 cells was confirmed in real-time PCR analysis, and the results revealed significant overexpression (368-fold) of MATE1 (versus mock cells) (data not shown). When the cellular uptake of MPP was measured in MATE1/HEK-293 cells and mock/HEK-293 cells, MPP uptake in MATE1/HEK-293 cells was higher than that in mock/HEK-293 cells (Table 1). Furthermore, in the presence of the MATE1 inhibitor, pyrimethamine (1 μM) (Ito et al., 2009), MPP uptake into MATE1/HEK-293 cells was completely inhibited. These results indicate that MATE1/HEK-293 cells used in the study were functional in cellular uptake of a MATE1 substrate. However, in contrast to MPP, differential uptake of EE2-Sul into MATE1/HEK-293 cells was not observed and pyrimethamine had no effect (Table 1). Under the conditions of the study, therefore, EE2-Sul did not behave as a MATE1 substrate.
Uptake of EE2-Sul by MRP4-Expressing Membrane Vesicles.
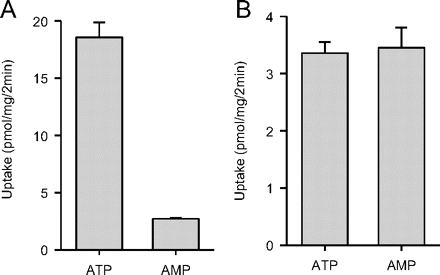
The potential for active transport of EE2-Sul was evaluated with insect cell (Sf9) membrane vesicles expressing MRP4. In this instance, the vesicles were shown to be functionally competent, because of ATP-dependent (versus AMP-dependent) estradiol-17β-d-glucuronide uptake (Fig. 5A). Under the same assay conditions, however, no ATP-dependent uptake of EE2-Sul was observed (Fig. 5B).
Uptake of estradiol 17β-d-glucuronide (A) and EE2-Sul (B) by Sf9 membrane vesicles expressing MRP4. Membrane vesicles were incubated with [3H]estradiol 17β-d-glucuronide (1 μM) or EE2-Sul (0.1 μM) at 37°C in the presence of 4 mM AMP or ATP. Vesicular uptake was determined as described under Materials and Methods. Results represent the mean ± S.D. of n = 3 determinations.
Discussion
After the oral administration of EE2, EE2-Sul circulates in the blood at high concentrations (6–23-fold greater than EE2) (Back et al., 1980). In addition, a significant amount of the EE2 dose is recovered in urine as EE2-Sul (Maggs et al., 1983). Although EE2 passive permeability is high (Zhang et al., 2007), due to its physicochemical properties, it is likely that EE2-Sul would require transporter-mediated uptake and efflux during the course of its elimination. Therefore, an attempt was made to evaluate the transporters that might be involved in its renal elimination. Because the localization of different renal transporters is known (Lee and Kim, 2004; Robertson and Rankin, 2006), it was possible to conduct EE2-Sul phenotyping studies with a panel of uptake transporters (e.g., OCT2, OAT1, and OAT3) known to be expressed on the basolateral membrane and efflux transporters (e.g., OAT4, MATE1, and MRP4) expressed on the brush border membrane of proximal tubule cells. Collectively, the data indicated that EE2-Sul behaves as an OAT3 and OAT4 substrate. Unfortunately, at the time of study, it was not possible to evaluate EE2-Sul as a substrate of additional renal transporters such as OATP4C1 (Mikkaichi et al., 2004).
Although various OCT family members are recognized as cation transporters, OCT1 and OCT2 have been shown to flux anionic compounds such as prostaglandin E2 and prostaglandin F2α (Kimura et al., 2002). However, prostaglandin structurally differs from EE2-Sul, and the latter did not serve as an OCT2 substrate (Table 1).
On the other hand, significant uptake of EE2-Sul was observed with OAT3 overexpressing HEK-293 cells (Fig. 1). A similar finding has been reported for other sulfate conjugates, such as estrone-3-sulfate (Cha et al., 2001; Tahara et al., 2005), DHEAS (Cha et al., 2001), and indoxyl sulfate (Deguchi et al., 2004). The Km value of EE2-Sul obtained in our experiments (21 μM) was slightly higher than that of estrone-3-sulfate (9.5 μM) (Tahara et al., 2005) and DHEAS (13 μM) (Nozaki et al., 2007). However, OAT1 uptake of EE2-Sul in OAT1-transfected cells was similar to that of vector-transfected cells (Table 1), which suggests that EE2-Sul is not an OAT1 substrate. The differentiation between OAT3 and OAT1 is similar to that reported for estrone-3-sulfate (Sweet et al., 2002) and DHEAS (Nozaki et al., 2007). Indoxyl sulfate serves as an OAT1 and OAT3 substrate (Deguchi et al., 2004).
Anionic drug transport in the kidney is more often than not OAT3-mediated, because the transporter is one of the major renal SLCs (Lee and Kim, 2004). Therefore, the results of the present study implicate OAT3 as a major player in the active (renal) uptake of circulating EE2-Sul. Because EE2-Sul was also found to be a substrate of OAT4, which is expressed on the brush border (apical) membrane of renal proximal tubules (Babu et al., 2002), it is assumed that OAT3 and OAT4 function in a coordinated manner to govern the overall renal-dependent elimination of the conjugate (Maggs et al., 1983). It is noteworthy that OAT4-mediated uptake into HEK-293 cells was described by a two-Km model (Km1 = 1.6 ± 1.8 μM; Km2 = 195 ± 138 μM) (Fig. 4). The Km (195 μM) of the low-affinity component is a best estimate, because the highest substrate concentration tested was 200 μM. Although this high Km component is an approximate value, the finding of two Kms for EE2-Sul is interesting, because all OAT4 substrates are known to exhibit single Km kinetics to date. In this regard, EE2-Sul as an OAT4 substrate is kinetically different from estrone-3-sulfate and DHEAS (Kimura et al., 2002). However, the overall transporter phenotype of estrone-3-sulfate (Cha et al., 2001; Tamai et al., 2001; Lee and Kim, 2004) resembles that of EE2-Sul (Han et al., 2010, this study), because OATP1B1-, OATP2B1-, and OAT3-mediated uptake is evident also. Furthermore, OAT4 is known to be an apical organic anion/dicarboxylate exchanger, and it functions as an apical pathway for the reabsorption of some organic anions in renal proximal tubules driven by an outwardly directed dicarboxylate gradient (Ekaratanawong et al., 2004). Consequently, OAT4-mediated secretion or reabsorption for EE2-Sul into the proximal tubule is possible.
Aside from OAT4 on the brush border membrane, other transporters on the brush border membrane of renal proximal tubule cells can be considered as EE2-Sul transporters. For example, MATE1 and MATE2-K are known to recognize some anionic compounds (e.g., acyclovir, ganciclovir, and estrone-3-sulfate), even though cationic drugs are favored as substrates (Tanihara et al., 2007; Terada and Inui, 2008). The results of the present study showed that EE2-Sul is not a MATE1 substrate (Table 1). Given that the specificity of MATE1 and MATE2-K is similar (Terada and Inui, 2008), it is unlikely that MATE2-K plays a role in the urinary excretion of EE2-Sul.
MRP4 has also been reported to be responsible for the renal secretion of some organic anions such as estradiol-17β-d-glucuronide (van Aubel et al., 2002), DHEAS (Zelcer et al., 2003), folic acid (Chen et al., 2002), and uric acid (Van Aubel et al., 2005). In our hands, however, no ATP-dependent uptake of EE2-Sul into MRP4-expressing membrane vesicles was evident. Likewise, consistent with the findings of Chu et al. (2004), EE2-Sul was not an MRP2 substrate (Han et al., 2010). As a result, the involvement of MRP2 and MRP4 in the secretion of EE2-Sul was ruled out. Because of ATP-dependent (fumitremorgin C-inhibited) EE2-Sul uptake in the presence of Sf9 membrane vesicles expressing BCRP (Han et al., 2010), and the considerable expression of BCRP in the kidney (Brown et al., 2008), it is likely that this second transporter is also involved in the renal elimination of EE2-Sul. Both OAT4 and BCRP exhibit similar two-Km EE2-Sul transport kinetics, with comparable low Km values (1.6 μM, OAT4; 2.9 μM, BCRP) (Table 2), and so may function in a coordinated manner.
Summary parameters describing the kinetics of EE2-Sul transport
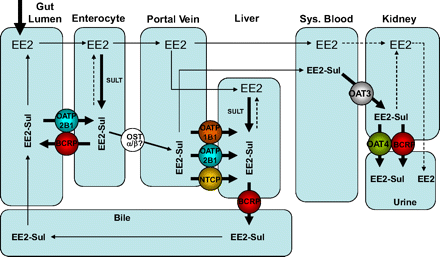
The results of previously described liver transporter phenotyping studies (Han et al., 2010) have shown that at least three anion transporters (OATP1B1, OATP2B1, and NTCP) may play a role in EE2-Sul liver uptake, whereas biliary secretion likely involves BCRP. Because renal elimination of EE2-Sul may involve OAT3, OAT4, and BCRP, enterohepatic cycling of EE2-Sul is governed by the complex interplay of numerous transporters (Fig. 6), with Kms that span a wide range (0.09–21 μM) (Table 2). At the same time, formation of EE2-Sul is mediated by sulfotransferases in both the intestine and liver, while conversion back to EE2 is catalyzed by sulfatases in each tissue (Zhang et al., 2007). Therefore, formation, distribution, and elimination of EE2-Sul are dependent on numerous enzymes and transporters (Fig. 6).
Simplified scheme describing EE2 disposition after oral administration with focus on transport of EE2-Sul. Once in the gut lumen, EE2 is absorbed (passively) and can undergo metabolism (e.g., sulfation to EE2-Sul) in enterocytes. BCRP can transport EE2-Sul back into the gut lumen. A considerable amount of EE2-Sul is also presented to the liver via the hepatic portal vein. In this instance, up to three transporters (OATP1B1, OATP2B1, and NTCP) can mediate uptake of EE2-Sul into the liver, where it is subjected to active efflux via canalicular BCRP. EE2-Sul can cycle back into the gut (via bile secretion) and be hydrolyzed to parent EE2, or it can be taken up by OATP2B1 therein. At the present time, the mechanism by which EE2-Sul undergoes efflux across the basolateral membrane (from the enterocyte into the portal blood) is unknown, but OSTα and OSTβ may be the candidate transporters (Han et al., 2010). The fraction of EE2-Sul that survives liver first pass can circulate, can be taken up by OAT3 in the kidney and secreted into urine via both OAT4 and BCRP. In tissues such as the gut, liver, and kidney, EE2-Sul may also be subjected to metabolism by sulfatases that yield parent EE2. SULT, sulfotransferase; OST, organic-solute transporter.
Although not the focus of the present study, EE2-Glu is also significantly excreted in the urine (Maggs et al., 1983). However, the transporters involved in EE2-Glu renal elimination have not been studied systematically. The uptake of EE2-Glu into the proximal tubule cells may occur via OAT3, as is the case with EE2-Sul, because OAT3 is able to transport glucuronide conjugates (Cha et al., 2001). Because OAT4 interacts preferentially with sulfate conjugates rather than glucuronide conjugates (Cha et al., 2000), the transport pathway of EE2-Glu may be different from that of EE2-Sul on the brush border membrane of proximal tubule cells, and the involvement of MRP2 is inferred (Chu et al., 2004). In addition, other efflux transporters such as BCRP and MRP4 may also interact with EE2-Glu, as in the case of estradiol-17β-d-glucuronide (Chen et al., 2003), SN-38-glucuronide (Nakatomi et al., 2001), and methylumbelliferone glucuronide (Suzuki et al., 2003).
EE2 has been in use for many years, and the original work of Maggs et al. (1983) described the recovery of various EE2 metabolites in the urine and bile of female subjects who received a radiolabel dose. At the time, the active secretion of EE2-Glu and EE2-Sul was suspected. With time, it has been possible to obtain additional information, and emerging data now point to the coordinated role of various drug-metabolizing enzymes and transporters that govern the metabolism and pharmacokinetics of EE2 itself, as well as the distribution and elimination of its major (conjugated) metabolites (Zhang et al., 2007; Han et al., 2010). Any one of the enzymes involved in EE2 metabolism, or transporters involved in EE2-Glu or EE2-Sul transport, could be subject to inhibition (or induction) by perpetrator drugs, or affected by polymorphisms (Zhou and You, 2007; Cusatis and Sparreboom, 2008; Maeda and Sugiyama, 2008). Such information is important, because most drug companies routinely conduct drug-drug interaction studies with oral contraceptive formulations that contain EE2. More often than not, the results of such studies have to be interpreted mechanistically.
Footnotes
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.031526.
-
ABBREVIATIONS:
- EE2
- 17α-ethinylestradiol
- EE2-Sul
- 17α-ethinylestradiol-3-O-sulfate
- EE2-Glu
- 17α-ethinylestradiol-3-O-glucuronide
- OAT
- organic anion transporter
- NTCP
- sodium-taurocholate cotransporting polypeptide
- BCRP
- breast cancer resistance protein
- SLC
- solute carrier
- ABC
- ATP-binding cassette
- OATP
- organic anion-transporting polypeptide
- OCT
- organic cation transporter
- MRP
- multidrug resistance-associated protein
- MATE1
- multidrug and toxin extrusion
- MDCK
- Madin-Darby canine kidney
- HEK
- human embryonic kidney
- PAH
- para-aminohippurate
- MPP
- 1-methyl-4-phenylpyridinium
- FRT
- Flp recombination target
- PAPS
- 3′-phosphoadenosine 5′-phosphosulfate
- HPLC
- high-performance liquid chromatography
- PCR
- polymerase chain reaction
- RQ
- relative quantification
- HBSS
- Hank's balanced salt solution
- BSP
- bromosulfophthalein
- DHEAS
- dehydroepiandrosterone-3-sulfate
- SN-38
- 7-ethyl-10-hydroxy-camptothecin.
- Received December 2, 2009.
- Accepted April 1, 2010.
- Copyright © 2010 by The American Society for Pharmacology and Experimental Therapeutics