Abstract
Phenacetin was withdrawn from the market because it caused renal failure in some patients. Many reports indicated that the nephrotoxicity of phenacetin is associated with the hydrolyzed metabolite, p-phenetidine. Acetaminophen (APAP), the major metabolite of phenacetin, is also hydrolyzed to p-aminophenol, which is a nephrotoxicant. However, APAP is safely prescribed if used in normal therapeutic doses. This background prompted us to investigate the difference between phenacetin and APAP hydrolase activities in human liver. In this study, we found that phenacetin is efficiently hydrolyzed in human liver microsomes (HLM) [CLint 1.08 ± 0.02 μl/(min · mg)], whereas APAP is hardly hydrolyzed [0.02 ± 0.00 μl/(min · mg)]. To identify the esterase involved in their hydrolysis, the activities were measured using recombinant human carboxylesterase (CES) 1A1, CES2, and arylacetamide deacetylase (AADAC). Among these, AADAC showed a Km value (1.82 ± 0.02 mM) similar to that of HLM (3.30 ± 0.16 mM) and the highest activity [Vmax 6.03 ± 0.14 nmol/(min · mg)]. In contrast, APAP was poorly hydrolyzed by the three esterases. The large contribution of AADAC to phenacetin hydrolysis was demonstrated by the prediction with a relative activity factor. In addition, the phenacetin hydrolase activity by AADAC was activated by flutamide (5-fold) as well as that in HLM (4-fold), and the activity in HLM was potently inhibited by eserine, a strong inhibitor of AADAC. In conclusion, we found that AADAC is the principal enzyme responsible for the phenacetin hydrolysis, and the difference of hydrolase activity between phenacetin and APAP is largely due to the substrate specificity of AADAC.
Introduction
Phenacetin [N-(4-ethoxyphenyl)acetamide] had been widely used as an analgesic antipyretic drug. Although phenacetin was developed as a prodrug of acetaminophen (APAP), it was withdrawn from the market because it was found to cause renal failure in some patients (Sicardi et al., 1991; Gago-Dominguez et al., 1999). Phenacetin is primarily metabolized to APAP through deethylation by CY1A2, but it was also metabolized to p-phenetidine through deacetylation by esterase in humans (Butler et al., 1989; Kudo et al., 2000). p-Phenetidine is further metabolized to N-hydroxyphenetidine, and this metabolite is considered to cause renal failure (Fig. 1) (Shudo et al., 1978; Vaught et al., 1981; Wirth et al., 1982). Studies on carcinogenic arylamines have implicated the metabolic conversion of N-hydroxylamines to nitroso derivatives as a critical pathway to the covalent binding to DNA (Hein et al., 1987; McManus, 1989). Thus, identification of the enzymes responsible for the phenacetin hydrolysis pathway is considered to be important to elucidate the phenacetin-induced renal failures.
Metabolic pathways of phenacetin.
APAP is well known to be biotransformed to quinoneimine or some other radical species, leading to hepatotoxicity (Moldéus et al., 1982; Dahlin et al., 1984). Moreover, p-aminophenol (PAP), the hydrolyzed metabolite of APAP, was reported to act as a nephrotoxicant in F344 rats, and inhibition of acetaminophen deacetylation by bis-(p-nitrophenyl) phosphate greatly diminished the nephrotoxicity by preventing the formation of PAP, suggesting that the nephrotoxicity of acetaminophen is due, at least in part, to PAP (Newton et al., 1982, 1985). However, in humans, there are no reports that the renal toxicity caused by APAP was associated with the production of PAP.
Esterases contribute to the hydrolysis of 10% of clinical therapeutic drugs including ester, amide, and thioester bonds (Williams et al., 2004). In particular, human carboxylesterase (CES), a major serine esterase, contributes to the hydrolysis of a majority of drugs and xenobiotics. The CES isoforms responsible for drug metabolism are CES1A1 (protein accession number: NP_001020365.1) and CES2 (NP_003860.2), but it was reported that these enzymes are not involved in phenacetin hydrolysis (Takai et al., 1997). As a candidate enzyme, human arylacetamide deacetylase (AADAC) (NP_001077.2) could be considered. Ross and Crow (2007) reported that when SDS-polyacrylamide gel electrophoresis gel of human liver microsomes (HLM) was treated with a fluorophosphate probe that reacts specifically with serine hydrolase enzymes, three enzymes, AADAC, CES1A1, and CES2, could be detected. Thus, AADAC, as well as CES enzymes, is a major serine hydrolase in HLM. AADAC was identified as the enzyme responsible for deacetylation of the carcinogen, 2-acetylaminofluorene (Probst et al., 1991), and we first demonstrated that AADAC is a principal enzyme in the hydrolysis of flutamide, an antiandrogen drug (Watanabe et al., 2009). In this study, we hypothesized that phenacetin is hydrolyzed by AADAC and investigated the difference in the catalytic efficiencies between phenacetin and APAP hydrolysis in HLM and AADAC.
Materials and Methods
Chemicals and Reagents.
Imidapril hydrochloride and imidaprilat were kindly supplied by Mitsubishi Tanabe Pharma Corporation (Tokyo, Japan). Flutamide, 5-amino-2-nitrobenzotrifluoride, phenacetin, APAP, and PAP were purchased from Wako Pure Chemicals (Osaka, Japan). p-Nitrophenyl acetate (PNPA) and p-phenetidine were purchased from Sigma-Aldrich (St. Louis, MO). Irinotecan hydrochloride (CPT-11) and 7-ethyl-10-hydroxycampothecin (SN-38) were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). HLM (pooled, n = 50) were purchased from BD Gentest (Woburn, MA). Primers were commercially synthesized at Hokkaido System Sciences (Sapporo, Japan). All other chemicals used in this study were of analytical or the highest quality commercially available.
Expression of Human AADAC in Sf21 Cells.
Expression of human AADAC using a Bac-to-Bac Baculovirus Expression System (Invitrogen, Carlsbad, CA) was performed according to the manufacturer's protocol. In brief, human AADAC cDNA was prepared by a reverse transcription-polymerase chain reaction technique using total RNA from human liver (Stratagene, La Jolla, CA) with the following primer sets: sense primer (5′-TAGAGACCAAGAAGCGGGAC-3′) and antisense primer (5′-GCTACATGTTTTACTATAGATTTTCC-3′). The polymerase chain reaction product was first subcloned into pTARGET Mammalian Expression Vector (Promega, Madison, WI). The AADAC cDNA in the pTARGET vector was then transferred into the pFastBac1 vector using the appropriate restriction enzymes. The pFastBac1 vector containing AADAC cDNA was transformed into DH10Bac competent cells, followed by transposition of the inserts into bacmid DNA. The sequence of the AADAC cDNA was determined using a Thermo Sequenase Cy5.5 Dye Terminator Cycle Sequencing kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK) with a Long-Read Tower DNA sequencer (GE Healthcare). In this study, the nucleotide sequence of AADAC was referred to as NM_001086.2. Nonrecombinant bacmid DNA (mock) was also prepared by the same procedures.
Spodoptera frugiperda Sf21 cells (Invitrogen) were grown in Sf-900 II SFM containing 10% fetal bovine serum at 27°C. The recombinant and mock bacmid DNAs were separately transfected into Sf21 cells with Cellfectin Reagent (Invitrogen), and the virus was harvested by collecting the cell culture medium at 72 h after transfection. Cells were routinely harvested 72 h after infection, washed twice with phosphate-buffered saline, and stored at 80°C until use. Cell homogenates were prepared by suspension in TGE buffer [10 mM Tris-HCl buffer (pH 7.4), 20% glycerol, and 1 mM EDTA (pH 7.4)] and by disruption by freeze-thawing three times according to the method reported by Ren et al. (2000). Then the suspensions were homogenized with a Teflon-glass homogenizer for 10 strokes. The enzyme preparations were stored at −80°C until use. The AADAC expression was confirmed with Western blotting according to the previous report (Watanabe et al., 2009). The protein concentrations were determined according to Bradford (1976).
Phenacetin Hydrolase Activity.
Phenacetin hydrolase activity was determined as follows. A typical incubation mixture (final volume of 0.2 ml) contained 100 mM potassium phosphate buffer (pH 7.4) and various enzyme sources (HLM and Sf21 cell homogenates expressing esterases: 0.4 mg/ml). In the preliminary study, we confirmed that the rate of p-phenetidine formation was linear with respect to the protein concentrations (<1.0 mg/ml human microsomal protein and Sf21 cell homogenates expressing esterases) and incubation time (<60 min). Phenacetin was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in the incubation mixture was 1.0%. The reaction was initiated by the addition of 0.05 to 4 mM phenacetin after a 2-min preincubation at 37°C. After the 20-min incubation at 37°C, the reaction was terminated by the addition of 10 μl of ice-cold 60% perchloric acid. After removal of the protein by centrifugation at 9500g for 5 min, a 50-μl portion of the supernatant was subjected to HPLC. The HPLC analysis was performed using an L-7100 pump (Hitachi, Tokyo, Japan), an L-7200 autosampler (Hitachi), an L-7405 UV detector (Hitachi), and a D-2500 chromato-integrator (Hitachi) equipped with a Mightysil RP-18 GP column (5-μm particle size, 4.6 mm i.d. × 150 mm; Kanto Chemical, Tokyo, Japan). The eluent was monitored at 232 nm with a noise-base clean Uni-3 (Union, Gunma, Japan), which can reduce the noise by integrating the output and increase the signal 3-fold by differentiating the output and 5-fold by further amplification with an internal amplifier, resulting in a maximum 15-fold amplification of the signal. The mobile phase was 10% acetonitrile containing 25 mM potassium dihydrogen phosphate. The flow rate was 1.0 ml/min. The column temperature was 35°C. The quantification of p-phenetidine was performed by comparing the HPLC peak height with that of an authentic standard. For kinetic analyses of phenacetin hydrolase activity, the parameters were estimated from the fitted curves using a computer program (KaleidaGraph; Synergy Software, Reading, PA) designed for nonlinear regression analysis.
To examine the esterase responsible for phenacetin hydrolysis, the stimulatory effect of flutamide on the phenacetin hydrolase activity was investigated. Flutamide was added to the reaction mixture described above at concentrations of up to 500 μM. Flutamide was dissolved in DMSO, and the final concentration of DMSO was 1.5% in all incubations. The phenacetin concentration was 1 mM. The control incubations were conducted without flutamide.
Moreover, inhibition analysis of phenacetin hydrolase activity was performed using eserine. The concentration of eserine ranged from 1 to 10 μM. The phenacetin concentration was 1 mM. Eserine was dissolved in distilled water, and the final concentration of DMSO in the incubation mixture was 1.0%.
Acetaminophen Hydrolase Activity.
The APAP hydrolase activity was determined as follows. A typical incubation mixture (final volume of 0.2 ml) contained 100 mM potassium phosphate buffer (pH 7.4), and various enzyme sources (HLM and Sf21 cell homogenates expressing esterases: 1.0 mg/ml). In the preliminary study, we confirmed that the rate of formation of PAP was linear with respect to the protein concentrations (<1.5 mg/ml human microsomal protein and Sf21 cells homogenate expressing esterases) and incubation time (<120 min). APAP was dissolved in DMSO and the final concentration of DMSO in the incubation mixture was 1.0%. The reaction was initiated by the addition of 0.5 to 25 mM APAP after a 2-min preincubation at 37°C. After the 60-min incubation at 37°C, the reaction was terminated by the addition of 100 μl of ice-cold methanol. After removal of the protein by centrifugation at 9500g for 5 min, a 50-μl portion of the supernatant was subjected to HPLC. The HPLC analysis was performed as described above. The eluent was monitored at 312 nm with a noise-base clean Uni-3. The mobile phase was 2% methanol containing 50 mM ammonium acetate. The flow rate was 1.0 ml/min The column temperature was 35°C. The quantification of PAP was performed by comparing the HPLC peak height with that of an authentic standard. The kinetic parameters were estimated as described above.
Other Hydrolase Activities.
PNPA, flutamide, imidapril, and CPT-11 hydrolase activities were determined as described previously (Takahashi et al., 2009; Watanabe et al., 2009; Maruichi et al., 2010). The substrate concentrations of PNPA, flutamide, imidapril, and CPT-11 were 200, 500, 100, and 2 μM, respectively.
Contribution of AADAC, CES1A1, and CES2 to Phenacetin Hydrolase Activity in HLM.
The percent contributions of AADAC, CES1A1, and CES2 to phenacetin hydrolase activity were estimated by applying the relative activity factor (RAF) as the ratio of activity values. The RAF values for AADAC (RAFAADAC) were determined as the ratios of the flutamide hydrolase activity in HLM to the values for recombinant AADAC. The RAF values for CES1A1 (RAFCES1A1) were determined as the ratios of the imidapril hydrolase activity in HLM to the values for recombinant CES1A1. The RAF values for CES2 (RAFCES2) were determined as the ratios of the CPT-11 hydrolase activity in HLM to the values for recombinant CES2. With use of RAF, the phenacetin hydrolase activities by AADAC, CES1A1, and CES2 in human liver microsomes (VAADAC, VCES1A1, and VCES2) were expressed as in eqs. 1 to 3:

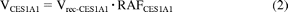
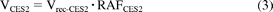 where Vrec-AADAC, Vrec-CES1A1, and Vrec-CES2 are the phenacetin hydrolase activities by recombinant AADAC, CES1A1, and CES2, respectively. The contributions of AADAC, CES1A1, and CES2 to the phenacetin hydrolase activity in HLM were calculated using eqs. 4 to 6:
where Vrec-AADAC, Vrec-CES1A1, and Vrec-CES2 are the phenacetin hydrolase activities by recombinant AADAC, CES1A1, and CES2, respectively. The contributions of AADAC, CES1A1, and CES2 to the phenacetin hydrolase activity in HLM were calculated using eqs. 4 to 6:
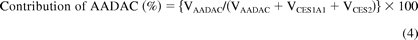
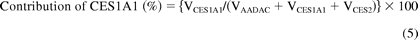

Statistical Analysis.
Comparisons of two and several groups were made with an unpaired, two-tailed Student's t test and a nonparametric analysis of variance, respectively. P < 0.05 was considered statistically significant.
Results
Kinetic Analyses of Phenacetin and APAP Hydrolase Activities in HLM.
To examine the catalytic efficiencies of phenacetin and APAP hydrolysis in HLM, both hydrolase activities were measured in pooled HLM (Fig. 2; Table 1). The maximum concentrations were 4 mM phenacetin and 25 mM APAP in the incubation mixture. For phenacetin hydrolysis, the Km and Vmax values in HLM were 3.30 ± 0.16 mM and 3.58 ± 0.20 nmol/(min · mg) protein, respectively, resulting in an intrinsic clearance (CLint) value of 1.08 ± 0.02 μl/(min · mg) protein (Fig. 2A). On the other hand, for the APAP hydrolysis, the maximum substrate concentration (25 mM) was not sufficiently high to determine the Km values because the solubility of APAP limited the substrate concentration that could be achieved in the incubation mixture (Fig. 2B). Therefore, the CLint value was calculated with the initial slope of the plots of velocity versus the substrate concentration. The CLint value of the APAP hydrolase activity in HLM was 0.02 ± 0.00 μl/(min · mg) protein, which was a value significantly (P < 0.001) lower than that of the phenacetin hydrolase activity. Therefore, the efficiency of phenacetin hydrolysis in HLM was much greater than that of APAP hydrolysis.
Kinetic analyses of phenacetin (A) and APAP hydrolase (B) activities in HLM. Each data point represents the mean ± S.D. of triplicate determinations.
Kinetic parameters for phenacetin and APAP hydrolase activities in HLM
PNPA, Flutamide, Imidapril, and CPT-11 Hydrolase Activities by Recombinant Human AADAC, CES1A1, and CES2.
We previously constructed the baculovirus expression systems of CES1A1 and CES2 (T. Fukami, S. Takahashi, N. Nakagawa, T. Maruichi, M. Nakajima, and T. Yokoi, submitted for publication). To confirm the substrate specificities of the homogenates of Sf21 cells expressing human AADAC, CES1A1, and CES2, the hydrolase activities of PNPA and substrates specific for each esterase were measured. The hydrolase activities of PNPA, a general esterase substrate, at a concentration of 200 μM by AADAC, CES1A1, and CES2 were 453.6 ± 32.2, 867.6 ± 21.1, and 364.7 ± 17.7 nmol/(min · mg) protein, respectively. Moreover, flutamide, imidapril, and CPT-11, which are typical substrates for AADAC, CES1A1, and CES2, respectively, were also measured (Takai et al., 1997; Humerickhouse et al., 2000; Watanabe et al., 2009). The flutamide hydrolase activity at a concentration of 500 μM was detected by AADAC [0.68 ± 0.03 nmol/(min · mg) protein] but not by CES1A1 and CES2. The imidapril hydrolase activity at a concentration of 100 μM was detected only by CES1A1 [1.73 ± 0.05 nmol/(min · mg) protein]. The CPT-11 hydrolase activity at a concentration of 2 μM was highly detected by CES2 [2.92 ± 0.09 pmol/(min · mg) protein] and slightly detected by AADAC [0.04 ± 0.00 pmol/(min · mg) protein]. These results indicate that the recombinant enzymes show specific catalytic functions of each isoform.
Kinetic Analyses of Phenacetin and APAP Hydrolase Activities by Recombinant AADAC, CES1A1, and CES2.
To identify the esterase responsible for phenacetin and APAP hydrolysis in HLM, both hydrolase activities were measured using recombinant AADAC, CES1A1, and CES2 (Fig. 3; Table 2). As shown in Fig. 3A, for phenacetin, the Km and Vmax values by recombinant AADAC were 1.82 ± 0.02 mM and 6.03 ± 0.14 nmol/(min · mg) protein, respectively, resulting in a CLint value of 3.32 ± 0.05 μl/(min · mg) protein. On the other hand, CES1A1 and CES2 showed lower Km values (0.27 ± 0.01 and 0.30 ± 0.02 mM, respectively) than AADAC but showed lower Vmax values [0.29 ± 0.02 and 0.38 ± 0.03 nmol/(min · mg) protein, respectively], with the result that the CLint value by AADAC was significantly (P < 0.001) higher than those by CES1A1 and CES2 [1.09 ± 0.01 and 1.25 ± 0.01 μl/(min · mg) protein, respectively]. Thus, all of the enzymes could catalyze the phenacetin hydrolysis, but AADAC showed a Km value similar to that in HLM (3.30 ± 0.16 mM) and the highest CLint value among the three enzymes. For APAP hydrolysis, the maximum substrate concentration (25 mM) was not sufficiently high to determine the Km values of CES1A1 and CES2. In AADAC, the Km and Vmax values could be calculated, but the calculated Km value (32.2 ± 1.3 mM) was unreliable because the value was higher than the maximum substrate concentration (25 mM). Therefore, the CLint values were calculated with the initial slope of the plots of velocity versus the substrate concentration. The values by AADAC, CES1A1, and CES2 were 0.07 ± 0.02, 0.01 ± 0.00, and 0.02 ± 0.00 μl/(min · mg) protein, respectively. Thus, the phenacetin and APAP hydrolase activities were detected by all enzymes, but their CLint values of APAP hydrolysis were much lower than those of phenacetin hydrolysis.
Kinetic analyses of phenacetin (A) and APAP hydrolase (B) activities by recombinant AADAC, CES1A1, and CES2. Each data point represents the mean ± S.D. of triplicate determinations.
Kinetic parameters for phenacetin and APAP hydrolase activities by recombinant AADAC, CES1A1, and CES2
Contributions of AADAC, CES1A1, and CES2 to Phenacetin Hydrolase Activity in HLM.
To predict the contributions of AADAC, CES1A1, and CES2 to the phenacetin hydrolase activity in HLM, RAF values for AADAC, CES1A1, and CES2 were calculated. It was reported that flutamide and imidapril are substrates specific for AADAC and CES1A1, respectively (Takai et al., 1997; Takahashi et al., 2009; Watanabe et al., 2009). On the other hand, it was reported that CPT-11 could be hydrolyzed by both CES1A and CES2, but the catalytic efficiency of CES2 is 60-fold higher than that of CES1A (Humerickhouse et al., 2000). In addition, the CPT-11 hydrolase activity at a substrate concentration of 2 μM in HLM was potently inhibited by loperamide, which is a selective inhibitor to CES2 (Quinney et al., 2005), with an inhibitor concentration to cause 50% inhibition (IC50) value of 0.46 μM (data not shown). Thus, we have judged that flutamide (500 μM), imidapril (100 μM), and CPT-11 (2 μM) could be used as the marker substrates for AADAC, CES1A1, and CES2, respectively. The value of flutamide hydrolase activity at 500 μM in HLM was 0.47 nmol/(min · mg) protein. By using the activity of recombinant AADAC [0.68 nmol/(min · mg) protein], the RAFAADAC value was calculated as 0.70. The value of imidapril hydrolase activity at 100 μM in HLM was 0.37 nmol/(min · mg) protein. By using the activity of recombinant CES1A1 [1.73 nmol/(min · mg) protein], the RAFCES1A1 value was calculated as 0.22. The value of CPT-11 hydrolase activity at 2 μM in HLM was 0.7 pmol/(min · mg) protein. By using the activity of recombinant CES2 [2.9 pmol/(min · mg) protein], the RAFCES2 value was calculated as 0.24.
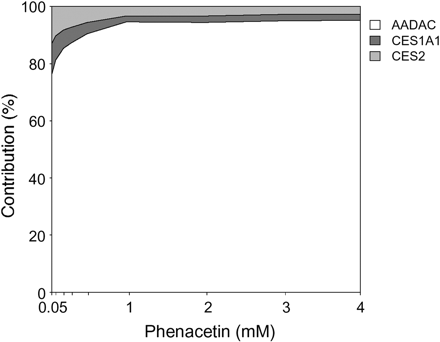
With use of the above RAF values, the contributions of AADAC, CES1A1, and CES2 to phenacetin hydrolysis in HLM were estimated according to eqs. 1 to 6 and are illustrated in Fig. 4. It was revealed that AADAC was the major enzyme that catalyzed the phenacetin hydrolysis in HLM with a contribution of 76.2 to 95.1% at phenacetin concentrations of 50 μM to 4 mM. Thus, it is suggested that, among the three esterases, AADAC contributed the most to phenacetin hydrolysis in HLM.
Percent contributions of AADAC (white), CES1A1 (dark gray), and CES2 (light gray) to phenacetin hydrolysis in HLM. Extrapolation was determined by RAF using HLM and recombinant AADAC, CES1A1, and CES2 from the homogenates of baculovirus-infected insect cells. Phenacetin concentrations used in this study were 0.05, 0.1, 0.2, 0.3, 0.5, 1.0, 2.0, 3.0, and 4.0 mM.
Effect of Flutamide on Phenacetin Hydrolase Activity in HLM and Recombinant AADAC, CES1A1, and CES2.
The phenacetin hydrolase activity in HLM was reported to be activated by flutamide (Kudo et al., 2000). In this study, it was confirmed that the phenacetin hydrolase activity in HLM was activated flutamide concentration dependently, with the result that approximately 5-fold higher activity occurred with the addition of 50 μM flutamide (Fig. 5). Thus, it is conceivable that an esterase responsible for the hydrolysis of phenacetin in HLM is activated by flutamide. Among phenacetin hydrolase activities by esterases investigated in this study, only the activity by AADAC was activated flutamide concentration dependently, and approximately 4-fold higher activity was shown by the addition of 50 μM flutamide. The activities by CES1A1 and CES2 were not activated by flutamide. Thus, these results suggested that the major esterase responsible for the phenacetin hydrolysis in HLM would be AADAC but not CES1A1 and CES2.
Effect of flutamide on phenacetin hydrolase activities in HLM, recombinant AADAC, CES1A1, and CES2. The phenacetin concentration was 1 mM. The control activities in HLM, recombinant AADAC, CES1A1, and CES2 were 1.32 ± 0.15, 2.25 ± 0.13, 0.16 ± 0.01, and 0.23 ± 0.02 nmol/(min · mg) protein, respectively. Each data point represents the mean ± S.D. of triplicate determinations.
Effects of Eserine on Phenacetin Hydrolase Activity in HLM and Recombinant AADAC, CES1A1, and CES2.
We previously reported that the flutamide hydrolase activity of AADAC was potently inhibited by 0.1 to 1 mM eserine (Watanabe et al., 2009). To further clarify that AADAC is mainly involved in the phenacetin hydrolysis in HLM, the inhibitory effect of eserine on the phenacetin hydrolase activities in HLM and recombinant AADAC, CES1A1, and CES2 was investigated (Fig. 6). The phenacetin hydrolase activities in HLM and AADAC were inhibited in an eserine concentration-dependent manner IC50 values of 4.08 ± 0.57 and 2.62 ± 0.13 μM, respectively. In contrast, the activities by CES1A1 and CES2 were not potently inhibited up to 5 μM eserine and were moderately inhibited by 10 μM eserine (residual activity: 57.6 ± 10.0 and 46.2 ± 4.3%, respectively). The similar inhibitory characteristics of HLM and AADAC also supported the fact that AADAC is the principal enzyme for the phenacetin hydrolysis in HLM.
Inhibitory effect of eserine on phenacetin hydrolase activity. Phenacetin hydrolase activities in HLM, recombinant AADAC, CES1A1, and CES2 were determined at a substrate concentration of 1 mM. The eserine concentrations used in this study were 1 to 10 μM. The control activity values of HLM, recombinant AADAC, CES1A1, and CES2 were 1.29 ± 0.03, 2.61 ± 0.21, 0.16 ± 0.01, and 0.36 ± 0.02 nmol/(min · mg) protein, respectively. Each data point represents the mean ± S.D. of triplicate determinations.
Discussion
Phenacetin had been widely used as an analgesic antipyretic, but it was withdrawn from the market because it was linked to cases of renal failure (Sicardi et al., 1991; Gago-Dominguez et al., 1999). There are many reports that the renal failure caused by phenacetin is associated with N-hydroxyphenetidine, the metabolite through hydrolysis, and the subsequent N-hydroxylation (Shudo et al., 1978; Vaught et al., 1981; Wirth et al., 1982). Thus, it is conceivable that phenacetin hydrolase played an important role in phenacetin-induced renal failure. In contrast, APAP, a primary metabolite of phenacetin, is well known to be biotransformed to quinoneimine or some other radical species, leading to hepatotoxicity (Moldéus et al., 1982; Dahlin et al., 1984), but APAP itself has been used as a clinical therapeutic drug. PAP, a hydrolyzed metabolite of APAP, was also demonstrated to be a nephrotoxicant in rat (Newton et al., 1982). APAP is known to cause renal failure in humans, although the risk is much lower than that for phenacetin (Buckalew, 1996). However, it is unknown whether PAP is associated with APAP-induced renal failures in humans.
In the present study, we found that the catalytic efficiency of phenacetin hydrolysis in HLM [CLint 1.08 ± 0.02 μl/(min · mg) protein] was significantly (P < 0.001) higher than that of APAP hydrolysis [CLint 0.02 ± 0.00 μl/(min · mg) protein] (Fig. 2). Although the phenacetin and APAP hydrolase activities were measured using human renal microsomes, both hydrolase activities were substantially low [0.27 ± 0.02 and 0.05 ± 0.00 nmol/(min · mg) protein at 4 mM phenacetin and 25 mM APAP, respectively] (data not shown). Thus, it was considered that the difference in the catalytic efficiencies between phenacetin and APAP hydrolysis in HLM might affect renal failure in humans.
We recently found that AADAC is a principal enzyme for flutamide hydrolysis (Watanabe et al., 2009). Flutamide is similar to phenacetin in chemical structure and molecular weight. Therefore, we considered that AADAC could be responsible for the phenacetin hydrolysis. However, CES is responsible for the hydrolysis of a majority of esterified drugs and xenobiotics, and the mRNA expression levels of CES1A1 and CES2 in human liver are 116- and 8-fold higher than that of AADAC (Watanabe et al., 2009). In this study, we found that the phenacetin hydrolase activity was detected by AADAC, CES1A1, and CES2. However, the Km value of AADAC (1.82 ± 0.02 mM) was similar to that of HLM (3.30 ± 0.16 mM). An Eadie-Hofstee plot of phenacetin hydrolase activity in HLM showed a curvature but not a biphasic pattern (Supplemental Fig. 1), although sigmoidicity was not obvious in the Michaelis-Menten plot (Fig. 2A). In addition, the contribution of AADAC (76.2–95.1%) was predicted to be higher than those of CES1A1 and CES2 (Fig. 4). Takai et al. (1997) reported that purified human CES1A and CES2 proteins could not hydrolyze phenacetin, although the phenacetin hydrolase activities by CES1A1 and CES2 were slightly detected in this study. The reason for the discrepancy is not clear, but it may partly be attributable to differences in the enzyme sources. The APAP hydrolase activities by AADAC, CES1A1, and CES2 were scarcely detected [CLint values 0.07 ± 0.01, 0.01 ± 0.00, and 0.02 ± 0.00 μl/(min · mg) of protein, respectively]. These values were substantially lower than those of the phenacetin hydrolase activities by AADAC, CES1A1, and CES2 (1.82 ± 0.02, 0.27 ± 0.01, and 0.30 ± 0.02 mM, respectively). Thus, the difference in the catalytic efficiencies between phenacetin and APAP hydrolysis in HLM is due to the substrate specificity of AADAC.
It has been reported that the phenacetin hydrolase activity in HLM was activated 4-fold by 300 μM flutamide (Kudo et al., 2000). To confirm the involvement of AADAC in the phenacetin hydrolysis in HLM, we examined whether the phenacetin hydrolase activity by AADAC was activated by flutamide (Fig. 5). The activities in HLM and AADAC were approximately 5- and 4-fold activated by 50 to 500 μM flutamide, respectively, whereas the activities by CES1A1 and CES2 were not activated. This result supported the fact that AADAC is a phenacetin hydrolase in HLM. This activation effect implies that AADAC has a binding site for flutamide to enhance the hydrolase activity. However, no activation effect of flutamide on the PNPA hydrolase activity by AADAC was observed (data not shown). Although we cannot clearly account for the difference, the activation of AADAC enzyme activity by flutamide would be observed using limited kinds of substrate. To further demonstrate that AADAC is the principle enzyme for phenacetin hydrolysis, the inhibitory effect of eserine, which is a potent inhibitor of AADAC (Watanabe et al., 2009), on phenacetin hydrolase activity was examined (Fig. 6). The phenacetin hydrolase activities in HLM and AADAC were inhibited in an eserine concentration-dependent manner with IC50 values of 4.08 ± 0.57 and 2.62 ± 0.13 μM, respectively. In contrast, the activities by CES1A1 and CES2 were poorly inhibited at up to 5 μM eserine and were moderately inhibited by 10 μM eserine (residual activity: 57.6 ± 10.0 and 46.2 ± 4.3%, respectively). Although eserine is also a potent inhibitor of butyrylcholinesterase, which is expressed in human plasma as well as liver (Iwatsubo, 1965; Li et al., 2005), the phenacetin hydrolase activity was not detected in human plasma (data not shown). These results also support the major contribution of AADAC to phenacetin hydrolysis in HLM.
In conclusion, we found that human AADAC is principally involved in phenacetin hydrolysis, and the difference in the catalytic efficiencies between phenacetin and APAP hydrolysis in HLM is due to the substrate specificity of AADAC. Thus, human AADAC would play an important role in phenacetin-induced renal failure.
Acknowledgments.
We acknowledge Mitsubishi Tanabe Pharma Corporation for kindly providing imidapril and imidaprilat. We thank Brent Bell for reviewing the manuscript.
Footnotes
This study was supported by the Ministry of Education, Science, Sports and Culture [Grant-in-Aid for Encouragement of Young Scientists 21790418].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.033720.
↵
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.-
ABBREVIATIONS:
- APAP
- acetaminophen
- PAP
- p-aminophenol
- CES
- carboxylesterase
- AADAC
- arylacetamide deacetylase
- HLM
- human liver microsomes
- PNPA
- p-nitrophenyl acetate
- CPT-11
- irinotecan hydrochloride
- SN-38
- 7-ethyl-10-hydroxycamptotecin
- DMSO
- dimethyl sulfoxide
- HPLC
- high-performance liquid chromatography
- RAF
- relative activity factor.
- Received April 2, 2010.
- Accepted June 11, 2010.
- Copyright © 2010 by The American Society for Pharmacology and Experimental Therapeutics