Abstract
In this study, the selectivity of UDP-glucuronosyltransferase (UGT) enzyme inhibition by ketamine (KTM) and the kinetics of KTM inhibition of human liver microsomal morphine (MOR) and codeine (COD) glucuronidation were characterized to explore a pharmacokinetic basis for the KTM-opioid interaction. With the exception of UGT1A4, KTM inhibited the activities of recombinant human UGT enzymes in a concentration-dependent manner. However, IC50 values were <100 μM only for UGT2B4, UGT2B7, and UGT2B15. UGT2B7 catalyzes MOR 3- and 6-glucuronidation and the 6-glucuronidation of COD, with an additional substantial contribution of UGT2B4 to the latter reaction. Consistent with the effects of KTM on the activities of recombinant UGT2B enzyme activities, KTM competitively inhibited human liver microsomal MOR and COD glucuronidation. Ki values for KTM inhibition of MOR 3- and 6-glucuronidation and COD 6-glucuronidation by human liver microsomes supplemented with 2% bovine serum albumin were 5.8 ± 0.1, 4.6 ± 0.2, and 3.5 ± 0.1 μM, respectively. Based on the derived inhibitor constants, in vitro-in vivo extrapolation was used to predict the effects of anesthetic and analgesic doses of KTM on MOR and COD clearances. Potentially clinically significant interactions (>50% increases in the in vivo area under the curve ratios) with MOR and COD were predicted for anesthetic doses of KTM and for a subanesthetic dose of KTM on COD glucuronidation.
Introduction
The N-methyl-d-aspartate receptor antagonist ketamine (KTM) has been used clinically as a dissociative anesthetic for more than four decades. However, KTM also exerts analgesic effects. In particular, there is evidence supporting the use of KTM as an adjuvant analgesic in several chronic pain states. KTM has been reported to provide improved pain relief in cancer patients who have a suboptimal analgesic response to high-dose morphine (MOR), with a concomitant reduction in opioid dose requirement and adverse effects (Bell 1999; Fitzgibbon and Viola, 2005). Although the improvement in MOR response due to KTM is generally believed to arise from attenuation of opioid tolerance and opioid-induced pain sensitivity after N-methyl-d-aspartate receptor blockade, it has been demonstrated recently that KTM inhibits the clearance of MOR via 3-glucuronidation in the isolated perfused rat liver preparation (Qi et al., 2010). Furthermore, KTM inhibited MOR 3-glucuronidation by rat liver microsomes.
As in the rat, MOR undergoes extensive hepatic glucuronidation in humans. Elimination by 3- and 6-glucuronidation comprise 57 and 10% of MOR systemic clearance, respectively (Hasselström and Säwe, 1993). UGT2B7 is the enzyme primarily responsible for hepatic MOR 3- and 6-glucuronidation (Stone et al., 2003). Like MOR, codeine (COD) is extensively glucuronidated in humans, with approximately 80% of the dose excreted in urine as codeine-6-β-d-glucuronide (C6G) (Yue et al., 1991). UGT2B7 also glucuronidates COD, although there is an additional substantial contribution of UGT2B4 to C6G formation (Raungrut et al., 2010).
To further explore a pharmacokinetic basis for the potentiation of opioid analgesia by KTM, we characterized the selectivity of UDP-glucuronosyltransferase (UGT) enzyme inhibition by KTM and the kinetics of KTM inhibition of human liver microsomal MOR and COD glucuronidation. We conducted inhibition studies with human liver microsomes (HLM) as the enzyme source in the presence and absence of bovine serum albumin (BSA; 2%). BSA sequesters long-chain unsaturated fatty acids released from the microsomal membrane during the course of an incubation, which act as potent inhibitors of UGT2B4 and UGT2B7 (Rowland et al., 2007, 2008; Raungrut et al., 2010). Thus, Ki values generated in the presence of BSA provide a more accurate prediction of drug-drug interaction potential in vivo (Rowland et al., 2006; Uchaipichat et al., 2006; Raungrut et al., 2010). Based on the derived inhibitor constants, in vitro-in vivo extrapolation (IV-IVE) was used to predict the likelihood of clinically significant interactions between KTM and MOR and COD.
Materials and Methods
Materials.
Alamethicin (from Trichoderma viride), COD, BSA, KTM, 4-methylumbelliferone (4MU), 4-methylumbelliferone β-d-glucuronide, morphine 3-β-d-glucuronide (M3G), and UDP-glucuronic acid (trisodium salt) were purchased from Sigma-Aldrich (Sydney, Australia); morphine hydrochloride (MOR) was from GlaxoSmithKline (Melbourne, Australia); C6G was from Toronto Research Chemicals (North York, ON, Canada); morphine 6-β-d-glucuronide (M6G) was from Salford Ultrafine Chemicals (Manchester, UK); and Supersomes expressing UGT2B4, 2B7, 2B15, and 2B17 were from BD Gentest (Woburn, MA). Lamotrigine (LTG) and lamotrigine N2-β-d-glucuronide were a gift from the Wellcome Research Laboratories (Beckenham, UK). Solvents and other reagents were of analytical reagent grade.
HLM and Recombinant UGT Proteins.
Human livers were obtained from the human “liver” bank of the Department of Clinical Pharmacology, Flinders Medical Centre (Adelaide, Australia). Approval for the use of human liver tissue in xenobiotic metabolism studies was obtained from the Flinders Clinical Research Ethics Committee. HLM were prepared by ultracentrifugation according to the method of Bowalgaha et al. (2005) and pooled by mixing equal protein amounts of microsomes from five livers (HL7, HL10, HL12, HL13, and HL40). HLM were activated by preincubation with alamethicin (50 μg/mg protein) on ice for 30 min before use (Boase and Miners, 2002). UGT1A cDNAs (namely, 1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, and 1A10) were stably expressed in a human embryonic kidney cell line (HEK293), as described previously (Uchaipichat et al., 2004). Due to the relatively low activity of UGT2B4, 2B7, 2B15, and 2B17 expressed in HEK293 cells, UGT2B enzymes expressed in insect cells (Supersomes) were used in inhibition studies.
Inhibition of Recombinant UGT Activities by Ketamine.
KTM inhibition of recombinant UGT enzyme activity was assessed using 4MU (UGT1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10, 2B7, 2B15, and 2B17), LTG (UGT1A4), and COD (UGT2B4) as the “probe” substrates. Incubation and analytical conditions were as described previously (Uchaipichat et al., 2004; Rowland et al., 2006; Raungrut et al., 2010). Substrate concentrations corresponded to the Km or S50 values for each enzyme/substrate combination. Concentrations of KTM used in the inhibition screening experiments were 0, 10, 100, 500, and 1000 μM. Data were compared with activities determined in the absence of KTM, and the inhibitory effects are reported as percentage of control activity for duplicate measurements.
Codeine 6-Glucuronidation and Morphine 3- and 6-Glucuronidation by Pooled HLM.
C6G formation by pooled HLM, in the absence and presence of 2% (w/v) BSA, was measured according to Raungrut et al. (2010). For studies of MOR glucuronidation, the incubation mixture (in a total volume of 200 μl) contained phosphate buffer (0.1 M, pH 7.4), MgCl2 (4 mM), pooled activated HLM (0.5 mg/ml), and MOR, in the absence and presence of 2% (w/v) BSA. After a 5-min preincubation, reactions were initiated by the addition of UDP-glucuronic acid (5 mM) and continued at 37°C in a shaking water bath for 30 min. Reactions were terminated by the addition of 2 and 8 μl of HClO4 (70%, v/v) for incubations performed in the absence and presence of 2% BSA, respectively. Samples were cooled on ice for 20 min and then centrifuged at 5000g for 10 min at 10°C. A 10-μl aliquot of the supernatant fraction was injected into the high-performance liquid chromatography (HPLC) column. HPLC was performed using an Agilent 1100 series instrument (Agilent Technologies, Sydney, Australia) fitted with a NovaPak C18 column (3.9 × 150 mm, 4-μm particle size; Waters, Milford, MA). Column eluant was monitored by fluorescence detection at excitation and emission wavelengths of 235 and 345 nm, respectively. The mobile phase, delivered at a flow rate of 1 ml/min, consisted of 100% acetonitrile in distilled water (A) and 1-octanesulfonic acid (4 mM), acetonitrile (5%), and glacial acetic acid (1%) in distilled water (B). Initial conditions were 4% phase A and 96% phase B followed by a linear gradient over 10 min to 9% phase A and 91% phase B, which was held constant for 1 min. Mobile phase A was then increased to 25%, which was held for 0.8 min, before returning to the starting conditions. Retention times for M3G, M6G, and morphine were 7.0, 10.3, and 13.6 min, respectively. Concentrations of M3G and M6G in incubation samples were quantified by comparison of peak areas to those of standard curves prepared over the concentration ranges of 0.5 to 20 and 0.5 to 5 μM, respectively.
Ketamine Inhibition of Human Liver Microsomal Morphine and Codeine Glucuronidation.
Inhibition of human liver microsomal (pooled) C6G, M3G, and M6G formation was determined at four KTM concentrations (see Fig. 2 for concentrations) at each of three COD or MOR concentrations (see Fig. 2) in the presence and absence of BSA (2% w/v), to determine mechanism of inhibition and Ki values. COD and MOR concentrations spanned the Km values for each substrate (Raungrut et al., 2010; N. Chau and J. O. Miners, unpublished results).
Nonspecific Binding of Ketamine to Human Liver Microsomes.
The binding of KTM to HLM and to HLM plus 2% BSA was characterized by equilibrium dialysis according to the general procedure of McLure et al. (2000). One side of dialysis cell contained KTM in phosphate buffer (0.1 M, pH 7.4), whereas the other side contained a suspension of either pooled HLM (1 mg/ml) or a combination of 2% BSA and HLM (1 mg/ml). We investigated KTM binding over the concentration ranges of 10 to 250 and 2 to 50 μM for samples containing HLM and HLM plus 2% BSA, respectively. After dialysis at 37°C for 4 h, a 200-μl aliquot was collected from each cell and treated with 500 μl of ice-cold methanol containing 4% glacial acid. Samples were chilled on ice for 20 min and subsequently centrifuged at 13,000g for 5 min at 4°C. An aliquot of the supernatant fraction was analyzed by HPLC. HPLC was performed using an Agilent 1100 series instrument (Agilent Technologies) fitted with a NovaPak C18 column (3.9 × 150 mm, 5-μm particle size; Waters). Mobile phase, delivered at flow rate 1 ml/min, comprised a 1:1 mixture of 30 mM phosphate buffer containing triethylamine (pH 7.2) and acetonitrile. Column eluant was monitored by UV absorbance at 215 nm. The retention time of KTM was 2.5 min. The KTM concentrations of dialysis samples were quantified by comparison of peak areas to those of a standard curve prepared over the concentration range of 2 to 250 μM. The unbound fraction of KTM in incubations (fuinc) was calculated as the drug concentration in the buffer compartment divided by the drug concentration in the protein compartment.
Data Analysis.
All data points represent the mean of duplicate estimates (<10% variance). Ki values for KTM inhibition of MOR and COD glucuronidation by pooled HLM were calculated using Enzfitter (Biosoft, Cambridge, UK). Expressions for competitive, uncompetitive, noncompetitive, and mixed inhibition were fit to experimental data. Goodness of fit was assessed from comparison of the F statistic, r2 values, S.E. of the parameter fit, and 95% confidence intervals. Ki values are reported as the parameter ± S.E. of the parameter estimate.
IV-IVE.
The predicted magnitude of the inhibition of MOR and COD hepatic clearance by KTM was calculated as the predicted ratios of the areas under the plasma concentration-time curves with (AUCi) and without (AUC) KTM coadministration
 where [I] is the inhibitor concentration, fm is the fraction of victim drug (COD or MOR) cleared along each pathway, and Ki is the inhibition constant generated in vitro. COD fractional clearance via 6-glucuronidation was taken as 80% (Yue et al., 1991), and the fractions of MOR 3- and 6-glucuronidation were 0.57 and 0.10, respectively (Hasselström and Säwe, 1993). The inhibitor concentration ([I]) in vivo was taken as either the total or unbound concentrations of KTM in plasma after anesthetic and analgesic doses (see Results and Discussion). The mean unbound fraction of KTM in the plasma of healthy subjects has been reported as 0.73 (Dayton et al., 1983).
where [I] is the inhibitor concentration, fm is the fraction of victim drug (COD or MOR) cleared along each pathway, and Ki is the inhibition constant generated in vitro. COD fractional clearance via 6-glucuronidation was taken as 80% (Yue et al., 1991), and the fractions of MOR 3- and 6-glucuronidation were 0.57 and 0.10, respectively (Hasselström and Säwe, 1993). The inhibitor concentration ([I]) in vivo was taken as either the total or unbound concentrations of KTM in plasma after anesthetic and analgesic doses (see Results and Discussion). The mean unbound fraction of KTM in the plasma of healthy subjects has been reported as 0.73 (Dayton et al., 1983).
Results and Discussion
The binding of KTM to HLM alone was negligible across the concentration range investigated (fuinc = 0.98 ± 0.02). However, binding of KTM to HLM plus 2% BSA was 21% (fuinc = 0.79 ± 0.02), which was independent of KTM concentration. The concentration of KTM added to incubations containing BSA was corrected for fuinc when calculating Ki values from inhibition studies. Previous results from this laboratory have demonstrated that COD and MOR do not bind significantly to HLM in the absence and presence of 2% BSA (Raungrut et al., 2010; N. Chau and J. O. Miners, unpublished data).
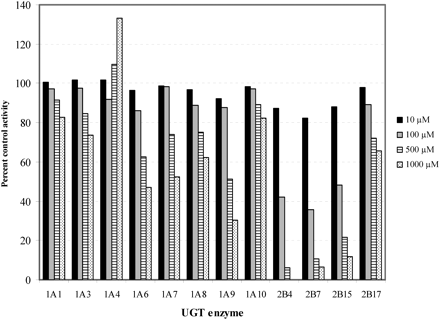
KTM inhibition of recombinant human UGT activities was assessed using 4-MU (UGT1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10, 2B7, 2B15, and 2B17), LTG (UGT1A4), and COD (UGT2B4) as the probe substrates. With the exception of UGT1A4, KTM inhibited all UGT enzymes in a concentration-dependent manner (Fig. 1). However, the greatest inhibition was observed with UGT2B4, 2B7, and 2B15 as the enzyme sources; respective estimated IC50 values were 69, 55, and 95 μM, whereas the IC50 values obtained for the other enzymes were an order of magnitude higher. As indicated under Materials and Methods, inhibition studies with the UGT2B enzymes were conducted with Supersomes as the enzyme source, whereas UGT1A enzymes were expressed in HEK293 cells. To exclude expression system-dependent effects of KTM, inhibition of UGT2B enzymes expressed in HEK293 cells (Uchaipichat et al., 2004) was also tested. Similar inhibition of UGT2B7 and UGT2B15 was observed (data not shown), although the low activity of UGT2B4 in HEK293 cell lysate precluded meaningful interpretation of inhibition data.
Effects of ketamine (0, 10, 100, 500, and 1000 μM) on the activities of recombinant human UGT enzymes. 4MU was used as the probe substrate, except for UGT1A4 (LTG) and UGT2B4 (COD). The 4MU, LTG, and COD concentrations correspond to the known Km or S50 values for each substrate/enzyme combination. Each bar represents the mean of duplicate measurements.
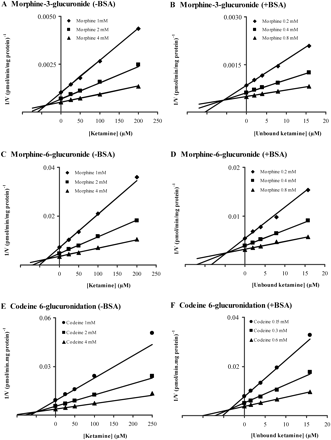
As noted under Introduction, UGT2B7 catalyzes COD and MOR glucuronidation whereas UGT2B4 additionally contributes to C6G formation. On the basis of the data shown in Fig. 1 and the previous report of KTM inhibition of MOR 3-glucuronidation in the rat (Qi et al., 2010), KTM inhibition of human liver microsomal MOR 3- and 6-glucuronidation and COD 6-glucuronidation was characterized kinetically. Effects of KTM on each of the three glucuronidation pathways were modeled well using the equation for competitive inhibition (Fig. 2). Ki values determined for MOR 3- and 6-glucuronidation and COD glucuronidation in the absence of BSA were 40 ± 0.7, 35 ± 0.7, and 52 ± 0.8 μM, respectively. Addition of BSA (2%) to incubations resulted in 85 to 93% reductions in Ki values; 5.8 ± 0.1, 4.6 ± 0.2, and 3.5 ± 0.1 μM for KTM inhibition of MOR 3- and 6-glucuronidation and COD 6-glucuronidation, respectively. As noted previously, we accounted for KTM binding to HLM plus 2% BSA in the calculation of Ki values.
Dixon plots for ketamine inhibition of COD 6-glucuronidation and MOR 3- and 6-glucuronidation by pooled HLM in the absence (A, C, E) and presence (B, D, F) of BSA (2%). Each point represents the mean of duplicate estimates whereas lines are from model fitting.
IV-IVE approaches have been applied successfully to predict in vivo clearance and inhibitory drug-drug interaction potential for compounds eliminated by glucuronidation (Miners et al., 2004, 2006, 2010). In particular, IV-IVE predicted the magnitude of the fluconazole-zidovudine and valproic acid-lamotrigine interactions (Rowland et al., 2006; Uchaipichat et al., 2006) and identified a number of potential interactions resulting from inhibition of COD 6-glucuronidation (Raungrut et al., 2010). Because these interactions arise from inhibition of UGT2B7 and UGT2B4, successful prediction of the change in the AUC of the victim drug requires the use of Ki values generated in the presence of BSA since sequestration of inhibitory long-chain unsaturated fatty acids is necessary to accurately measure an inhibitor constant. It is noteworthy that the decrease in the Ki for KTM inhibition of C6G glucuronidation observed in the presence of BSA (52 to 3.5 μM) was larger than the approximate 7-fold reduction in Kis for MOR 3- and 6-glucuronidation. The larger decrease in the Ki for COD glucuronidation presumably reflects differential effects of long-chain unsaturated fatty acids (and hence BSA) on UGT2B4 and UGT2B7; both enzymes contribute to C6G formation (Raungrut et al., 2010), whereas UGT2B7 is the dominant enzyme responsible for MOR 3- and 6-glucuronidation (Stone et al., 2003).
Predicted effects of KTM on MOR and COD clearances via glucuronidation were determined for anesthetic and analgesic doses. A mean KTM plasma concentration of 9.3 μM was reported during steady-state anesthesia after induction with 2 mg/kg and a maintenance dose of approximately 40 μg · kg−1 · min−1 (Idvall et al., 1979). When used as an adjuvant analgesic, subanesthetic doses of KTM are typically administered by subcutaneous infusion, but plasma concentrations have not been reported when KTM is administered in this manner. However, a peak KTM plasma concentration of 2.7 μM has been observed in patients administered 0.5 mg/kg epidurally (Xie et al., 2003). After low-dose (0.125–0.250 mg/kg i.v.) administration, Clements and Nimmo (1981) found that a KTM concentration of >0.42 μM (100 μg/l) was required for analgesia (defined as pain relief for >5 min).
Substitution of the above values (for [I]) in eq. 1 predict approximate 60 and 140% increases in the AUCs for MOR and COD, respectively, after an anesthetic dose of KTM (Table 1). Potentially clinically significant inhibition of COD clearance was also predicted for a subanesthetic dose given epidurally (54% increase in AUC ratio), but a lesser effect was predicted for the MOR AUC. No interaction was predicted for low dose KTM given intravenously. As expected, smaller increases in AUCs were predicted when unbound KTM concentrations were used for IV-IVE. Previous studies of drug-drug interaction potential have generally reported optimal prediction of the AUC ratio using total drug concentration (e.g., Ito et al., 2004; Rowland et al., 2006).
Predicted fold increase in the AUCs of codeine and morphine based on plasma ketamine concentrations reported following anesthetic and analgesic doses
In summary, data presented here demonstrate that KTM inhibits human UGT2B4, UGT2B7, and UGT2B15. Consistent with the known involvement of UGT2B4 and UGT2B7 in MOR and COD metabolism, KTM inhibited the glucuronidation of these compounds by HLM. Ki values generated in the presence of BSA predicted potential inhibition of opioid clearance after anesthetic and possibly subanesthetic doses of KTM, supporting the hypothesis that a pharmacokinetic mechanism may contribute to KTM-opioid interactions. Furthermore, KTM may potentially precipitate interactions with other compounds because UGT2B7 contributes to the metabolism of numerous other drugs, including anticancer agents and nonsteroidal anti-inflammatory drugs, and endogenous compounds such hydroxy-steroids (Jin et al., 1997; Kiang et al., 2005; Miners et al., 2010).
Authorship Contributions
Participated in research design: Uchaipichat, Raungrut, Janchawee, Evans, and Miners.
Conducted experiments: Uchaipichat, Raungrut, and Chau.
Performed data analysis: Uchaipichat and Miners.
Wrote or contributed to the writing of the manuscript: Uchaipichat, Evans, and Miners.
Other: Evans and Miners acquired funding for the research.
Footnotes
This study was supported by a grant from the National Health and Medical Research Council of Australia. V.U. was the recipient of an Australian Education International Endeavour Fellowship, and P.R. was supported in part by a Prince of Songkla University Graduate Studies grant.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.039727.
-
ABBREVIATIONS:
- KTM
- ketamine
- MOR
- morphine
- COD
- codeine
- C6G
- codeine-6-β-d-glucuronide
- UGT
- UDP-glucuronosyltransferase
- HLM
- human liver microsomes
- BSA
- bovine serum albumin
- IV-IVE
- in vitro-in vivo extrapolation
- 4MU
- 4-methylumbelliferone
- M3G
- morphine 3-β-d-glucuronide
- M6G
- morphine 6-β-d-glucuronide
- LTG
- lamotrigine
- HEK
- human embryonic kidney
- HPLC
- high-performance liquid chromatography
- fuinc
- unbound fraction of KTM in incubations
- AUC
- area under the plasma concentration-time curve.
- Received March 24, 2011.
- Accepted May 6, 2011.
- Copyright © 2011 by The American Society for Pharmacology and Experimental Therapeutics