Abstract
Antibody drug conjugates are emerging as a powerful class of antitumor agents with efficacy across a range of cancers; therefore, understanding the disposition of this class of therapeutic is crucial. Reported here is a method of enriching a specific organelle (lysosome) to understand the catabolism of an anti-CD70 Ab-MCC-DM1, an antibody drug conjugate with a noncleavable linker. With such techniques a higher degree of concentration-activity relationship can be established for in vitro cell lines; this can aid in understanding the resultant catabolite concentrations necessary to exert activity.
Introduction
Antibody drug conjugates (ADCs) are agents that consist of a cytotoxic drug conjugated to a targeting antibody through various types of linkers (Alley et al., 2010; Erickson et al., 2010). A major design feature of an ADC is maximum delivery of the cytotoxic agent to target tumor tissue and minimized delivery of the cytotoxin to untargeted tissues. The concept of treating cancer with ADCs gained momentum with the approval by the FDA of brentuximab vedotin (SGN-35, Adcetris) for the treatment of Hodgkin’s lymphoma and anaplastic large cell lymphoma, and more recently, with the approval of trastuzumab emtansine (T-DM1, Kadcyla) for the treatment of human epidermal growth factor receptor-2 (HER2)–positive cancers (de Claro et al., 2012; Amiri-Kordestani et al., 2014).
Essentially there are two broad classes of ADC linkers; those that are chemically or enzymatically labile, referred to as cleavable, and those that are chemically stable, referred to as noncleavable. Cleavable (chemically labile) linkers are designed so that the ADC stays intact in circulation but releases the cytotoxin upon internalization by the target cell, usually owing to a change in chemical environment in the cell or specific proteases in the cell that cleave the cytotoxin from the antibody (Fishkin et al., 2011; Han and Zhao, 2014). Subsequent tumor cell lysis results in ADC catabolites (cytotoxins) capable of permeating adjacent cells (bystander effect), which can be desirable in tumor environments with heterogeneous expression of the target antigen (Kovtun et al., 2006).
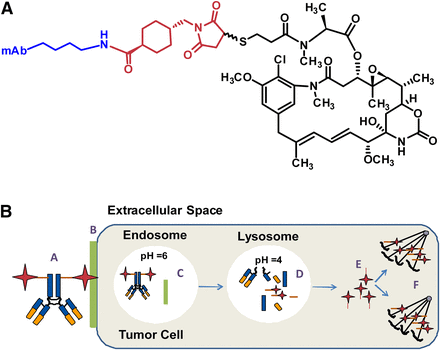
An ADC with a noncleavable linker does not contain chemical functionalities that are readily susceptible to intracellular degradation, either in circulation or inside the target cell (Fig. 1A). The general mechanism of action for an ADC with a noncleavable linker requires the following (Fig. 1B): 1) binding to the extracellular target/antigen, 2) endocytosis and trafficking to a lysosome, 3) degradation of the ADC, and 4) intracellular release of the active cytotoxin specie(s) where the mechanism of action of the cytotoxin (e.g., binding microtubulin) leads to cell death. The effectiveness of the cytotoxin can be tuned by aligning physical-chemical properties of the linker, but in general the cytotoxins have low permeability and rapid clearance, which limits exposure to healthy tissues.
(A) General structure of noncleavable antibody maytansinoid conjugate. (B) Mechanism of ADC activity, where: A, localization of ADC to the tumor cell; B, binding of ADC to specific antigen; C, internalization into the endosome release the antigen from ADC; D, catabolism of ADC in the lysosome; E, release of Lys-MCC-DM1 into the cytoplasm; and F, binding of Lys-MCC-DM1to tubulin resulting in cell cycle arrest and apoptosis.
Antibody drug conjugates designed with noncleavable linkers generally use a stable thioether, N-succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC), with the maytansinoid moiety most commonly being, N2′-deacetyl-N2-(3-mercapto-1-oxopropyl)-maytansine (DM1) (Erickson and Lambert, 2012). Conjugation of the cytotoxin occurs through the ἑ-amino group lysine residues. Notably, this technology was used for Kadcyla, where DM1 is linked to trastuzumab (a humanized monoclonal antibody) by a thioester bond between the sulfhydryl of DM1 and maleimide of SMCC (Lambert and Chari, 2014; Peddi and Hurvitz, 2014).
In theory for an ADC with the noncleavable linker (SMCC), one main catabolite should be released after cellular processing, lysine-Nἑ-MCC-DM1 (Lys-MCC-DM1). The formation of this single catabolite has been shown in vitro by dosing cells with 3[H]-ADC, and it was proposed that the formation of this catabolite occurs in the lysosome (Erickson et al., 2006, 2012).
Presented here, is the development of a sensitive mass spectroscopy method to eliminate the need for radiolabeled ADC for investigation of in vitro catabolism. In addition, this work conclusively demonstrates that catabolism of anti-CD70 Ab-MCC-DM1 (Fig. 1A) to the active catabolite Lysine-MCC-DM1 occurs in the lysosomal compartment.
Materials and Methods
Materials.
Bafilomycin A1 was purchased from ACROS Organics (West Chester, PA). Lys-MCC-DM1 was obtained from ImmunoGen (Waltham, MA). Anti-CD70 Ab-MCC-DM1 and anti-ST Ab-MCC-DM1 (control antibody) were obtained from Amgen, Inc. (Thousand Oaks, CA), and the concentration of anti-CD70 Ab-MCC-DM1 was stoichiometrically calculated to the moles of DM1 attached to the antibody (DM1 equivalents). All commercial solvents (Sigma-Aldrich, St. Louis, MO) were of liquid chromatography–mass spectrometry analytical grade. Lysosome Enrichment Kit for tissue and cultured cells was purchased from Thermo Scientific (Waltham, MA). 786-0 Cells were originally obtained from ATCC (Manassas, VA) and passaged in RPMI + 10% fetal bovine serum. All other materials were purchased from Sigma-Aldrich, unless specified.
Cellular Activity of ADC-Treated 786-0 Cells.
786-0 Cells were cultured in a 96-well tissue culture plate and incubated at 37°C, 5% CO2 for approximately 4–6 hours. Serial dilutions of anti-CD70 Ab-MCC-DM1 were added to wells, as well as a control conjugate (no antigen binding). Following a 96-hour exposure, cellular ATP level, which correlates to cell number, was assessed using the CellTiter Glo reagent (Promega, Madison, WI). Luminescence was measured using an EnVision plate reader (PerkinElmer, Waltham, MA).
Lysosome Enrichment from ADC-Treated 786-0 Cells.
ADC-treated 786-0 cells from a 6-well plate were trypsinized and combined with trypsinized untreated 786-0 cells from a T175 flask. The cells were centrifuged, the supernatant aspirated, and pellet washed with cold phosphate-buffered saline. The cells were centrifuged once more, the supernatant aspirated, and pellet resuspended in homogenization buffer (with added protease inhibitors). The cell suspension was then transferred to a Dounce tissue grinder and homogenized on ice by performing 180 strokes to achieve about 80–90% cell breakage. The lysate was centrifuged to remove unbroken cells and nuclei. The resulting postnuclear supernatant was diluted in OptiPrep gradient media to a final concentration of 15% OptiPrep media. A discontinuous density gradient was prepared in descending concentrations (30%, 27%, 23%, 20%, 17%). The sample containing 15% OptiPrep media was layered on top of the gradient and sample ultracentrifuged at 55,000 rpm for 2 hours. The lysosome band was removed and frozen for further analysis by mass spectrometry.
Sample Preparation and Analytical Method for Measurement of ADC Catabolite.
Each sample (total cell lysate or enriched lysosome fraction) was prepared by adding 1 ml of lysis buffer (50 mM Tris-HCl, 100 mM NaCl, 0.1% Triton X-100, pH 7.4), and the sample was transferred to a 15-ml glass conical test tube. Ethyl acetate (1 ml containing 1 nM internal standard, DM4) was then added to extract catabolites, a total of three times, and each aliquot was transferred to a new glass conical test tube. Ethyl acetate extractions were completely dried under nitrogen. Samples were reconstituted in 50:50 mixture of water/acetonitrile (50 μl) before analysis by liquid chromatography–tandem mass spectroscopy, which was performed using a Thermo Scientific Quantum (TSQ) triple quadrupole mass analyzer in line with a Thermo Accela Ultra Performance Liquid Chromatography separation system (Thermo Scientific, Waltham, MA). Analytes were separated on a Kinetex 2.6-μm C18 50 × 2.1-mm column. The peaks eluted using a linear gradient at 0.2 ml/min and mobile phases A: 0.1% formic acid in water and B: 0.1% formic acid in acetonitrile. The gradient started at 80% A (0–0.5 minutes). Solvent B was linearly increased from 20% at 0.5 minutes to 100% at 4 minutes. The total run time was 6 minutes. The precursor-to-fragment ions monitored were Lys-MCC-DM1 (1103 > 485 m/z) and DM4 (795 > 547 m/z). The TSQ mass analyzer was operated at unit mass resolution (full width at half maximum set to 0.7 for both Q1 and Q3). The ion-transfer capillary temperature was set at 325°C. The other electrospray ionization source parameters for each instrument were optimized for flow rate of 0.2 ml/min. The lower limit of quantification for the method was 0.5 pg/ml. Data acquisition and analysis were accomplished with Xcalibur Software version 1.4 (Thermo Scientific).
Results
In Vitro Potency of Anti-CD70 Ab-MCC-DM1 in 786-0 Cells and Formation of Lys-MCC-DM1 in Total Cell Lysate.
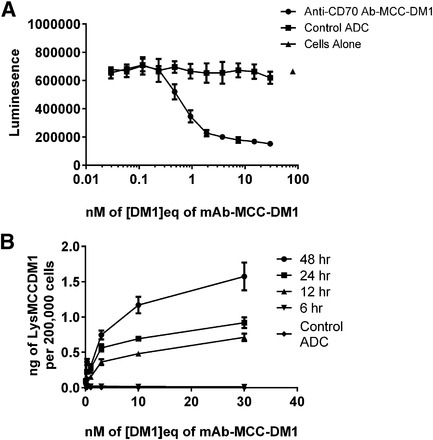
Anti-CD70 Ab-MCC-DM1 [0–30 nM (DM1 equivalents)] was assayed for its cytotoxic potency against CD70-positive 786-0 cells. The ADC inhibited cell growth with an approximate IC50 value of 1 nM (DM1 equivalents) upon a 96-hour exposure (Fig. 2A). In contrast to anti-CD70 Ab-MCC-DM1, the control conjugate exhibited no cell growth inhibition. In a similar experimental setup, the total cellular Lys-MCC-DM1 was measured over a time course (Fig. 2B) following exposure to varying concentrations of anti-CD70 Ab-MCC-DM1. The results indicated that the formation of Lys-MCC-DM1 was both concentration- and time-dependent. Measurable amounts of Lys-MCC-DM1 were not detected until 12 hours after addition of anti-CD70 Ab-MCC-DM1. Control conjugate generated no measurable amounts of Lys-MCC-DM1 over 48 hours.
(A) Serial dilutions of anti-CD70 Ab-MCC-DM1 were added to 786-0 cells, as well as a control ADC (no antigen binding). Following a 96-hour exposure cell-growth inhibition was assessed using the CellTiter Glo reagent. The total luminescence, which correlates to cell number, is plotted against concentration of anti-CD70 Ab-MCC-DM1 (DM1 equivalents). (B) Accumulation of Lys-MCC-DM1 in 786-0 cells treated with varying concentrations of anti-CD70 Ab-MCC-DM1 [0–30 nM (DM1 equivalents)] over 48 hours. The total amount (ng) of Lys-MCC-DM1 per approximately 200,000 cells is plotted against time.
Effect of a Lysosomal Inhibitor on Cellular Toxicity Induced by Anti-CD70 Ab-MCC-DM1.
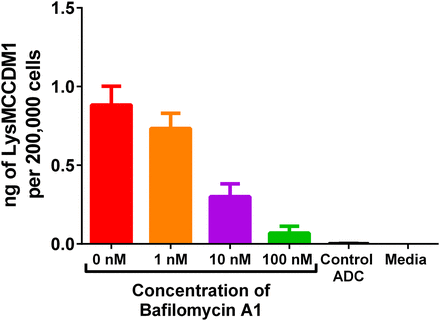
To examine whether uptake and processing of anti-CD70 Ab-MCC-DM1 through the lysosomes was necessary for the activity against 786-0 cells, cellular toxicity was measured in the presence of varying concentrations of bafilomycin A1. Bafilomycin A1 selectively inhibits V-ATPase, a proton pump present in the endosomes and lysosomes, which leads to neutralization of the pH in these vesicles (Bowman et al., 1988). A dose-response for cellular activity was observed over a concentration range of 0–100 nM bafilomycin A1 (Supplemental Fig. 1). The concentration of Lys-MCC-DM1 formed was also measured in the same experiment and the results correlated to the dose response observed in the cellular activity assay (Fig. 3). These findings provide mechanistic support for the importance of lysosomal processing in the activation of anti-CD70 Ab-MCC-DM1.
Formation of Lys-MCC-DM1 in the presence of bafilomycin (0–100 nM) and 10 nM (DM1 equivalents) of anti-CD70 Ab-MCC-DM1 for 24 hours.
Enrichment of Lysosome Fractions from Anti-CD70 Ab-MCC-DM1-Treated 786-0 Cells.
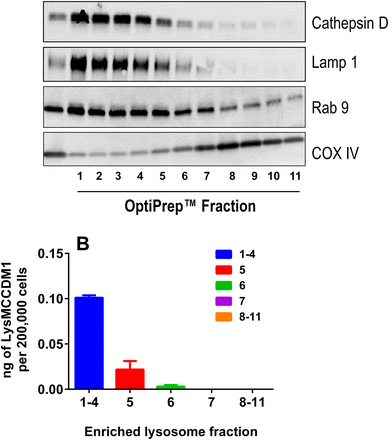
To further investigate the role of lysosomal processing in the catabolism of anti-CD70 Ab-MCC-DM1, enrichment and isolation of the lysosomal fraction was performed. After enrichment for the lysosomal fraction, the concentration of Lys-MCC-DM1 was measured by liquid chromatography–tandem mass spectroscopy. The concentration of Lys-MCC-DM1 correlated well with the markers of lysosomal activity (cathepsin D, Lamp1, and Rab9; Fig. 4) in fractions 1–5. The total amount of Lys-MCC-DM1 found in the lysosomal fractions was 0.12 ng/200,000 cells, in comparison with the total cell lysate, where the amount was 0.69 ng/200,000 cells. Therefore, 17% of the total amount of Lys-MCC-DM1 resides in the lysosome 24 hours after treatment with the anti-CD70 Ab-MCC-DM1. The remaining Lys-MCC-DM1 was accounted for in the cellular supernatant and extraction from the cell pellet after lysosomal enrichment, with total recoveries ranging from 89 to 94% (Supplemental Table 1).
(A) Analysis of subcellular fractions from 786-0 cells. Western blots show lysosome/endosome enrichment (cathepsin D, Lamp1, and Rab 9) in top fractions with minimum contamination from mitochondria (COX IV). (B) Amount of Lys-MCC-DM1 measured in enriched lysosomes fractions after 10 nM [DM1 equivalents] anti-CD70 Ab-MCC-DM1 treatment of 24 hours.
Discussion
Antibody drug conjugates are emerging as a powerful class of antitumor agents (Wakankar et al., 2011; Lin et al., 2013). Understanding the site of catabolism and possible rate-limiting steps in the cellular distribution of the cytotoxic catabolite are crucial, especially for ADCs with noncleavable linkers that are dependent on cellular catabolism processes to generate the cytotoxin (Doronina et al., 2006; Erickson et al., 2006, 2010). Reported here is the catabolism of anti-CD70 Ab-MCC-DM1, an ADC with a noncleavable linker (SMCC). To investigate the catabolism, 786-0 cells were treated with an antigen-specific anti-CD70 Ab MCC-DM1. High resolution mass spectroscopy scanning methods coupled with multiple mass defect filtering were used to search for catabolites. Only one catabolite was detected, Lysine-MCC-DM1 (data not shown). Moreover, the total Lys-MCC-DM1 concentrations correlated with cell-growth inhibition.
Treatment of cells with a known lysosomal protease inhibitor bafilomycin indicated the importance of the lysosomal compartment in the breakdown of anti-CD70 Ab-MCC-DM1, similar to a previous report using a different antibody-MCC-DM1 molecule. To further the understanding of the formation of Lys-MCC-DM1, enrichment of the lysosomes from the cellular components was performed. Although this is a well-established technique (Gjoen et al., 1997), this is the first report of enrichment of the lysosomal fraction after catabolism of an ADC for identification of catabolites. No Lys-MCC-DM1 was detectable outside the cell in the supernatant, consistent with the low permeability of Lys-MCC-DM1 as measured in LLC-PK1 cells (Supplemental Table 2). This finding showcases a distinction of this class of noncleavable ADC, little potential for bystander effect owing to sequestration of the Lys-MCC-DM1 in the cell.
Previously, it was reported that Lys-MCC-DM1 is a circulating species in serum, in both preclinical ADME studies as well as in the clinic for T-DM1, an ADC with the same linker-drug combination studied here (Shen et al., 2012). With numerous other ADCs in clinical trials with noncleavable linkers (Sassoon and Blanc, 2013), understanding the mechanism in which Lys-MCC-DM1 is formed is essential. Given that ADCs are complex molecules composed of both large and small molecule entities, the findings here add to the overall understanding of cellular disposition of an ADC with a noncleavable linker. Furthermore, experimental design with this method can be used to derive a link between ADC catabolism and resultant catabolite concentrations necessary to exert activity.
Authorship Contributions
Participated in research design: Rock, Rock, Patel, Hamblett, Tometsko, Fanslow.
Conducted experiments: B. Rock, Patel, Tometsko.
Performed data analysis: B. Rock, Tometsko, Hamblett.
Wrote or contributed to the writing of the manuscript: Rock, Rock.
Footnotes
- Received March 26, 2015.
- Accepted June 18, 2015.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- ADC
- antibody drug conjugate
- anti-CD70 Ab-MCC-DM1
- antibody-N-succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate- N2-deacetyl-N2-(3-mercapto-1-oxopropyl)-maytansine
- DM1
- N2′-deacetyl-N2-(3-mercapto-1-oxopropyl)-maytansine
- DM4
- N2′-deactyl-N2′-(4-mercapto-4-methylo-1-oxopentyl)-maytansine
- Lys-MCC-DM1
- Lysine-Nἑ-4-(N-maleimidomethyl)cyclohexane-1-carboxylate)-N2-deacetyl-N2-(3-mercapto-1-oxopropyl)-maytansine
- SMCC
- N-succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics