In the above article [Pearson D, Weiss HM, Jin Y, van Lier JJ, Erpenbeck VJ, Glaenzel U, End P, Woessner R, Eggimann F, and Camenisch G (2017) Drug Metab Dispos 45:817–825; DOI: https://doi.org/10.1124/dmd.117.075358], it was discovered that the purity/drug content of the reference standard for the acyl glucuronide metabolite was previously over estimated. Consequently, a correction factor of 0.70 was applied to revise these concentrations in several places in the article as provided below.
In Table 1, last column, line 2, “1450 ± 353” should be “1020 ± 247,” and in line 3, “9840 ± 1770” should be “6890 ± 1240.” The corrected Table 1 is provided below.
PK parameters of total radioactivity in whole blood and plasma and of fevipiprant and its main circulating metabolite (AG metabolite) in plasma
Values are mean ± SD
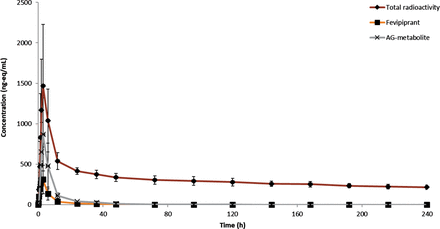
Fig. 2 shows a correction in the concentration of the AG metabolite. A decrease as a correction factor of 0.70 was applied. The corrected figure is provided below.
Plasma concentrations of fevipiprant and AG metabolite, and total radioactivity after oral administration of a 200 mg dose of [14C]-fevipiprant to four healthy subjects. Units for fevipiprant and AG metabolite concentrations are ng/ml, for total radioactivity concentrations ng-eq/ml. Concentrations of fevipiprant and AG metabolite were determined by LC-MS/MS, and concentrations of total radioactivity were determined by LSC.
In the Results section, under the subsection on Metabolite Identification and Profiles, and Metabolite PK Analysis, “87.7%” should be “90.4% in the third paragraph.
In Table 3, last column, line 2, “27.6 ± 3.53” should be “19.25 ± 2.48.” The corrected Table 3 is provided below.
Fevipiprant and metabolites in excreta
The HTML and PDF versions of the article have been corrected.
The authors apologize for any inconvenience this may have caused.
- Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics