Visual Overview
Abstract
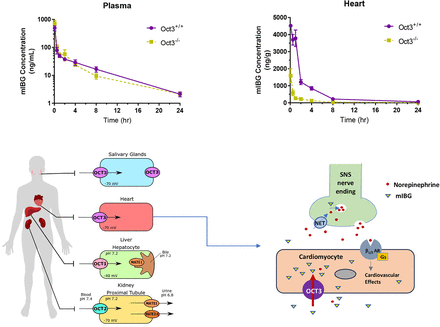
Heart failure (HF) is a chronic disease affecting 1%–2% of the global population.123I-labeled meta-iodobenzylguanidine (mIBG) is US Food and Drug Administration-approved for cardiac imaging and prognosis risk assessment in patients with HF. As a norepinephrine analog, mIBG is believed to be transported into adrenergic nerve terminals by the neuronal norepinephrine transporter (NET) and hence image sympathetic innervation of the myocardium. We previously showed that mIBG is an excellent substrate of organic cation transporter 3 (OCT3), an extraneuronal transporter expressed in cardiomyocytes. Here, we evaluated the in vivo impact of Oct3 on mIBG disposition and tissue distribution using Oct3 knockout mice. Oct3+/+ and Oct3−/− mice were administered with mIBG intravenously, and mIBG plasma pharmacokinetics and tissue exposures were determined. In Oct3+/+ mice, mIBG exhibited extensive accumulation in multiple tissues (heart, salivary gland, liver, and adrenal gland). No difference was observed in overall plasma exposure between Oct3+/+ and Oct3−/− mice. Strikingly, cardiac mIBG was depleted in Oct3−/− mice, resulting in 83% reduction in overall cardiac exposure (AUC0–24 h: 12.7 vs. 2.1 μg × h/g). mIBG tissue exposure (AUC0–24 h) was also reduced by 66%, 36%, and 31% in skeletal muscle, salivary gland, and lung, respectively, in Oct3−/− mice. Our data demonstrated that Oct3 is the primary transporter responsible for cardiac mIBG uptake in vivo and suggested that cardiac mIBG imaging mainly measures OCT3 activity in cardiomyocytes but not NET-mediated uptake in adrenergic nerve endings. Our findings challenge the current paradigm in interpreting cardiac mIBG imaging results and suggest OCT3 as a potential genetic risk marker for HF prognosis.
SIGNIFICANCE STATEMENT 123I-labeled meta-iodobenzylguanidine is used for cardiac imaging and risk assessment in heart failure patients. Contrary to the current belief that meta-iodobenzylguanidine (mIBG) tracks cardiac sympathetic innervation due to its uptake by the neuronal norepinephrine transporter, the authors demonstrated that cardiac mIBG uptake is mediated by the extraneuronal transporter Oct3. Their findings warrant a re-evaluation of the scientific rationale behind cardiac mIBG scan and further suggest organic cation transporter 3 as a risk factor for disease progression in heart failure patients.:
Footnotes
- Received February 29, 2024.
- Accepted May 23, 2024.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants R01-GM066233 and T32-GM007750] and the National Center for Advancing Translational Sciences [Grant TL1-TR002318].
The authors declare no potential conflicts of interest.
- Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics
DMD articles become freely available 12 months after publication, and remain freely available for 5 years.Non-open access articles that fall outside this five year window are available only to institutional subscribers and current ASPET members, or through the article purchase feature at the bottom of the page.
|