Abstract
Troglitazone, a new oral antidiabetic drug, is reported to be mostly metabolized to its conjugates and not to be oxidized by cytochrome P-450 (P-450) enzymes. Of fourteen cDNA-expressed human P-450 enzymes examined, CYP1A1, CYP2C8, CYP2C19, and CYP3A4 were active in catalyzing formation of a quinone-type metabolite at a concentration of 10 μM troglitazone, whereas CYP3A4 had the highest catalytic activity at 100 μM substrate. In human liver microsomes, rates of the quinone-type metabolite formation (at 100 μM) were correlated well with rates of testosterone 6β-hydroxylation (r = 0.98), but those at 10 μM troglitazone were not correlated with any of several marker activities of P-450 enzymes. Quercetin efficiently inhibited quinone-type metabolite formation (at 10 μM troglitazone) in human samples that contained relatively high levels of CYP2C, whereas ketoconazole affected these activities in liver microsomes in which CYP3A4 levels were relatively high. Anti-CYP2C antibodies strongly inhibited quinone-type metabolite formation (at 10 μM troglitazone) in CYP2C-rich human liver microsomes (by ∼85%); the intensity of this effect depended on the human samples and their P-450 status. The results suggest that in human liver both CYP2C8 and CYP3A4 have major roles in quinone-type metabolite formation and that the hepatic contents of these two P-450 forms determine which P-450 enzymes play major roles in individual humans. CYP3A4 may be expected to play a role in formation of quinone-type metabolite from troglitazone even at a low concentration in humans.
Cytochrome P-450 (P-450)1 comprises a superfamily of enzymes that catalyze oxidation of a great number of xenobiotic chemicals such as drugs, toxic chemicals, and carcinogens as well as endobiotic chemicals, including steroids, fatty acids, prostaglandins, and lipid-soluble vitamins (Guengerich and Shimada, 1991; Guengerich, 1995). In human livers, levels of each of the P-450 forms and roles in various substrate oxidations vary. CYP3A4 is the major P-450 enzyme involved in the oxidation of a large number of compounds (Wrighton and Stevens, 1992; Gonzalez and Gelboin, 1994; Guengerich, 1995).
Troglitazone (Noscal or Rezulin) is a new oral antidiabetic drug recently approved in Japan and the United States for use in the treatment of noninsulin-dependent diabetes mellitus (Sparano and Seaton, 1998). Troglitazone biotransformation has been investigated in rats, mice, dogs, monkeys, and humans, and it is reported to be metabolized mainly to the conjugates shown in Fig.1, the sulfate (metabolite 1) and glucuronide (metabolite 2) (Izumi et al., 1997a,b; Kawai et al., 1998). In humans, the major products found in plasma are metabolite 1 and, to a lesser extent, a quinone-type metabolite (metabolite 3) (Physicians’ Desk Reference, 1999). Metabolite 3 also was detected in monkeys to a similar extent as in humans but not in rats and mice. Sex differences in pharmacokinetics are observed in rats, i.e., females showed a higher plasma concentration of troglitazone and a lower concentration of metabolite 1 than males, and they excrete a female-specific metabolite, a hydroxylated metabolite 1 (metabolite 4), in the bile (Odaka et al., 1995; Kawai et al., 1997). The oxidized metabolite 3 was found to be further processed to the sulfate in rats (Kawai et al., 1997). Although troglitazone is reported not to be metabolized by human CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4, it has been reported to be a potential inducer of CYP3A4 at clinically relevant concentrations (Physicians’ Desk Reference, 1999) and to significantly decrease cyclosporine (Kaplan et al., 1998), terfenadine, and ethynylestradiol (Koup et al., 1998; Physicians’ Desk Reference, 1999) concentrations in humans. With regard to roles of P-450 enzymes involved in troglitazone metabolism, there is (only) one report that rat CYP2C12 has been shown to catalyze the hydroxylation of metabolite 1 to metabolite 4 (Odaka et al., 1995). Roles of P-450 enzymes in the oxidation of troglitazone to the quinone-type metabolite 3 in human liver are not clear.
Proposed metabolic pathways of troglitazone.
Rare cases of severe idiosyncratic hepatocellular injury during marketed use of troglitazone have been reported (Watkins and Whitcomb, 1998; Physicians’ Desk Reference, 1999). The hepatic injury is usually reversible, but very rare cases of hepatic failure, leading to death or liver transplant, have been reported. Injury has occurred after both short- and long-term troglitazone treatment in the United States (Physicians’ Desk Reference, 1999) and after troglitazone treatment for a period ≥4 weeks in Japan (Kuramoto et al., 1998). Hepatic toxicity of troglitazone was not observed in any experimental animals tested, including monkeys, which showed similar metabolite profiles as humans (Summary Basis for Approvals, 1997). In general, quinone-type metabolites are considered to be active intermediates in drug-induced hepatic toxicity after metabolic activation in examples such as acetaminophen, halothane, and diclofenac (Pumford and Halmes, 1997; Bort et al., 1999). It is important to elucidate the mechanism of hepatic toxicity of troglitazone for its safe use. The present study was, therefore, undertaken to determine which P-450 enzymes are most effective in the formation of the quinone-type metabolite (metabolite 3) of troglitazone. Initial studies were performed with recombinant human P-450 enzymes in different expression systems and further studies were done with human liver microsomes to put the work into perspective.
Materials and Methods
Chemicals.
Troglitazone and its metabolites were kindly provided by Sankyo (Tokyo, Japan). Testosterone, paclitaxel, tolbutamide,S-mephenytoin, and their metabolites and reagents used in this study were obtained from sources described previously or were of highest qualities commercially available (Shimada and Yamazaki, 1998).
Enzyme Preparations.
Human liver microsomes were prepared in 10 mM Tris-HCl buffer (pH 7.4) containing 0.10 mM EDTA and 20% glycerol (v/v) as described previously (Guengerich, 1994). Liver samples HL-3, -4, and -10 corresponded to those designated HL-110, -111, and -136 (Guengerich, 1995) and HL-C6, -C15, and -C19 (Yamazaki et al., 1998), respectively. These three microsomal preparations contained total spectrally determined P-450 levels (nanomoles per milligram microsomal protein) of 0.53, 0.32, and 0.45, respectively. Microsomal sample HL-3 had CYP2C9, CYP2C19, and CYP3A4 levels of 12, 0.7, and 73% total P-450, respectively, as judged by immunoblot analysis. Sample HL-4 contained CYP2C9, CYP2C19, and CYP3A4 levels of 21, 3.4, and 14%, respectively, and sample HL-10 contained 16, 1.3, and 46% total P-450, respectively. Rabbit NADPH-P-450 reductase (Guengerich et al., 1981) and human cytochromeb5 (b5; Shimada et al., 1986) were purified from liver microsomes by the methods described. Recombinant CYP3A4 was purified from Escherichia coli membranes as described elsewhere (Gillam et al., 1993). CYP3A4/reductase membranes of E. coli in which CYP3A4 and NADPH-P-450 reductase cDNAs had been introduced were prepared as described previously (Parikh et al., 1997). Recombinant P-450 enzymes expressed in microsomes of insect cells infected with baculovirus containing human P-450 and rabbit or human NADPH-P-450 reductase cDNA inserts (Baculosomes or Supersomes) were obtained from PanVera (Madison, WI) or Gentest (Woburn, MA), respectively. Recombinant CYP3A4 in lymphoblastoid cells coexpressing NADPH-P-450 reductase was obtained from Gentest. The P-450 contents were used as described in the data sheets provided by the manufacturers. Anti-rat CYP2C13 and anti-rat CYP3A2 IgG for immunoinhibition experiments with human liver microsomes were obtained from Daiichi Pure Chemicals (Tokyo, Japan). According to the manufacturer’s data sheets, these anti-CYP2C IgG and anti-CYP3A IgG preparations inhibited >80% of paclitaxel 6α-hydroxylation, diclofenac 4′-hydroxylation, and S-mephenytoin 4′-hydroxylation and testosterone 6β-hydroxylation, respectively, in human liver microsomes and did not show cross-reactivity.
Enzyme Assays.
The standard incubation mixture (final volume of 0.20 ml) contained human liver microsomes (1.0 mg/ml), 100 mM Tris-HCl buffer (pH 7.4), an NADPH-generating system consisting of 0.5 mM NADP+, 5 mM glucose 6-phosphate, 0.5 U of glucose 6-phosphate dehydrogenase per milliliter, and troglitazone (10–100 μM). In some cases, microsomes or membranes containing recombinant P-450 enzymes (0.025 μM) coexpressing P-450 reductase were used. In reconstitution systems, purified CYP-3A4 (0.025 μM), P-450 reductase (0.050 μM) and b5 (0.025μM), lipid mixture, and cholate were used in the presence of an NADPH-generating system or 0.1 mM cumene hydroperoxide (CuOOH) (Yamazaki et al., 1995). Incubations were carried out at 37°C for 20 min and terminated by adding 0.20 ml of ice-cold C2H5OH. After centrifugation at 900g for 10 min, product formation in the supernatant was determined by HPLC with a C18 (5 μm) analytical column (4.6 × 150 mm, YMC-Pack A-302; YMC Co. Ltd., Kyoto, Japan). The elution was conducted with a mixture of 42% CH3CN/0.05% H3PO4 (v/v) at a flow rate of 2.0 ml/min and detection was by UV absorbance at 230 nm (Kawai et al., 1998).
Activities of paclitaxel 6α-hydroxylation, tolbutamide methyl hydroxylation, S-mephenytoin 4′-hydroxylation, and testosterone 6β-hydroxylation were determined as described (Brian et al., 1989; Walle, 1996) with slight modifications (Inoue et al., 1997; Shimada and Yamazaki, 1998).
Other Assays.
Concentrations of P-450 and b5 (Omura and Sato, 1964) and protein (Lowry et al., 1951) were estimated as described. The contents of P-450 enzymes in human liver microsomes were estimated by coupled SDS-polyacrylamide gel electrophoresis/immunochemical development (Western blotting) (Guengerich et al., 1982).
Kinetic analysis for substrate oxidations by P-450 enzymes were estimated with a computer program (KaleidaGraph; Synergy Software, Reading, PA) designed for nonlinear regression analysis.
Results
Metabolite 3 Formation Catalyzed by Recombinant P-450 Enzymes Expressed in Different Expression Systems.
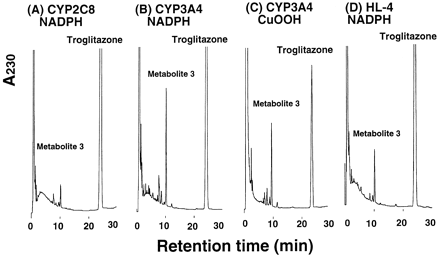
Formation of metabolite 3 from troglitazone was investigated with recombinant P-450 enzymes coexpressed with NADPH-P-450 reductase in the presence of an NADPH-generating system or CuOOH. Typical chromatograms are shown in Fig. 2. After incubation of troglitazone with CYP2C8, CYP3A4 (Supersomes; Gentest), and human liver microsomes (a sample of HL-4), formation of metabolite 3 was observed. CYP3A4-mediated formation of metabolite 3 from troglitazone also was confirmed with CuOOH as an oxygen surrogate (Fig. 2C); nonenzymatic conversion of troglitazone to metabolite 3 by CuOOH was not observed.
Representative HPLC chromatograms of troglitazone metabolites catalyzed by recombinant CYP2C8 (A), CYP3A4 (B and C), and human liver microsomes (a sample HL-4, D).
Troglitazone (30 μM) was incubated for 20 min at 37°C with CYP2C8 or CYP3A4 (0.025 μM), and human liver microsomes (1.0 mg/ml) in the presence of an NADPH-generating system. In (C), troglitazone was incubated with CYP3A4 for 5 min in the presence of 0.1 mM CuOOH instead of the NADPH-generating system.
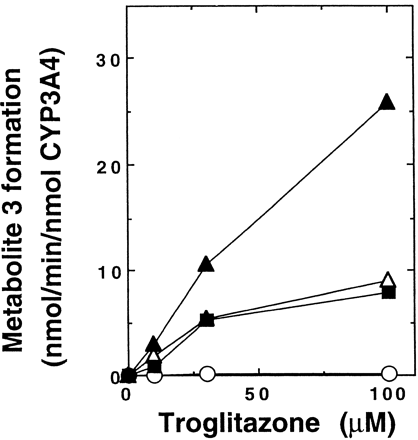
Because troglitazone has been reported not to be metabolized by recombinant CYP3A4 (Physicians’ Desk Reference, 1999), we investigated CYP3A4-catalyzed metabolite 3 formation in different expression systems (Fig. 3). Microsomes of lymphoblastoid cells containing CYP3A4 coexpressed with P-450 reductase showed little metabolite 3 formation, although CYP3A4 expressed in two kinds of insect cell microsomes with baculovirus systems or in E. coli membranes catalyzed metabolite 3 formation in the presence of an NADPH-generating system. In a reconstituted system containing purified CYP3A4, metabolite 3 formation was observed in the presence of NADPH or CuOOH (Table 1). Rates for metabolite 3 formation in the reconstituted systems were lower than those in insect cell microsomes expressing CYP3A4, P-450 reductase, andb5 (Supersomes; Gentest).
Metabolite 3 formation from troglitazone catalyzed by recombinant CYP3A4 systems.
Troglitazone (10–100 μM) was incubated for 20 min with different CYP3A4 systems (0.025 μM) coexpressing NADPH-P-450 reductase in the presence of an NADPH-generating system. Microsomes of lymphoblastoid cells (○) containing CYP3A4:reductase = 1:1 (Gentest), microsomes of insect cells with baculovirus system (▵) containing CYP3A4:reductase = 1:4.6 (Baculosomes; PanVera), microsomes of insect cells with baculovirus system (▴) containing CYP3A4:reductase:b5 = 1:12:16 (Supersomes; Gentest), and E. coli membranes (▪) containing CYP3A4:reductase = 1:1.1 were used.
Rates of quinone-type metabolite 3 formation from troglitazone catalyzed by recombinant CYP3A4 in the presence of an NADPH-generating system or CuOOH
Metabolite 3 Formation Catalyzed by Recombinant P-450 Enzymes Expressed in Baculovirus Systems.
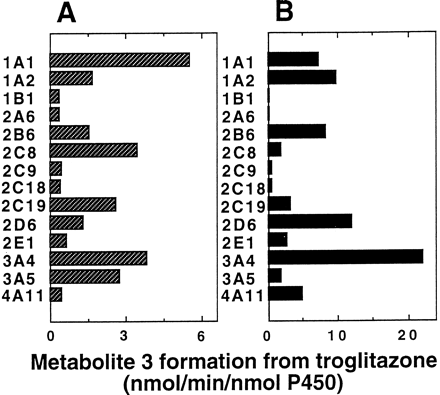
Fourteen forms of recombinant human P-450 enzymes expressed in baculovirus systems with human NADPH-P-450 reductase were used to compare which P-450 forms are active in catalyzing metabolite 3 formation at substrate concentrations of 10 and 100 μM (Fig.4) (selected on the basis of Km = 28 μM in liver microsomes vide infra). At 10 μM troglitazone, CYP1A1, CYP3A4, CYP2C8, CYP3A5, and CYP2C19 were highly active in converting troglitazone to metabolite 3 (Fig. 4A). All cDNA-expressed human P-450 enzymes examined had some measurable activity. When the substrate concentration was increased to 100 μM, CYP3A4 had the highest catalytic activity (Fig. 4B).
Metabolite 3 formation from troglitazone at 10 (A) or 100 μM (B) catalyzed by recombinant P-450 enzymes.
Troglitazone was incubated for 20 min with recombinant P-450 systems (0.025 μM P-450, Supersomes; Gentest) coexpressing NADPH-P-450 reductase in the presence of an NADPH-generating system.
The above-mentioned results suggest that the major P-450 enzymes involved in the troglitazone oxidation in human liver may be CYP2C and CYP3A. Kinetic analysis of the troglitazone oxidation by recombinant P-450 enzymes showed that CYP2C8, CYP2C9, CYP2C19, and CYP3A4 had respective Km values of 2.7, 3.6, 3.1, and 120 μM and Vmax values of 4.2, 0.6, 2.8, and 47 nmol/min/nmol P-450 (Table 2).
Kinetic analysis for quinone-type metabolite 3 formation from troglitazone by recombinant P-450 enzymes
Characterization of Quinone-Type Metabolite 3 Formation in Human Liver Microsomes.
Formation of metabolite 3 from troglitazone in standard reaction mixtures containing human liver microsomes was increased linearly with microsomal protein concentration up to 2.0 mg /ml and with time up to 30 min (Fig. 5). Metabolite 3 formation was increased in a substrate concentration-dependent manner, with a hyperbolic plot and some inhibition at high concentrations.
Effects of microsomal protein (A), incubation time (B), and substrate concentration (C) on quinone-type metabolite 3 formation catalyzed by human liver microsomes (sample HL-4).
The basic incubation mixture was used. In (A) and (B), the concentration of troglitazone was 100 μM; in (B) and (C), the microsomal protein concentration was 1.0 mg/ml; in (A) and (C), the mixtures were incubated for 20 min in the presence of an NADPH-generating system.
Correlations between marker drug oxidation activities and rates of metabolite 3 formation from troglitazone were analyzed with 10 human liver microsomal samples (Fig. 6). Two substrate concentrations, 10 (Fig. 6, A–D) and 100 μM (Fig. 6E), were used. Activities of paclitaxel 6α-hydroxylation, tolbutamide methyl hydroxylation, S-mephenytoin 4′-hydroxylation, and testosterone 6β-hydroxylation did not show good correlations with activities of troglitazone metabolite 3 formation at 10 μM concentrations in these liver microsomes. However, at high substrate concentration (100 μM), metabolite 3 formation activities were highly correlated (r = 0.98) with the testosterone 6β-hydroxylation activities.
Relationship between rates for metabolite 3 formation from troglitazone at 10 (A–D) or 100 μM (E) and drug oxidation activities in different human liver microsomes.
Kinetic analysis of metabolite 3 formation in human liver microsomal samples HL-4 and HL-10, which had relatively high contents of CYP2C enzymes (Yamazaki et al., 1998), revealed apparent singleKm and Vmaxvalues of 29 μM and 143 pmol/min/mg protein and 28 μM and 281 pmol/min/mg protein, respectively (Fig.7). HL-3, with a relatively high content of CYP3A4 (Yamazaki et al., 1998), gave a curve with positive cooperativity and apparently high Km (435 μM) and Vmax (3040 pmol/min/mg protein) values.
Kinetic analysis for metabolite 3 formation by human liver microsomal samples.
Troglitazone (1–200 μM) was incubated for 20 min with human liver microsomes (1.0 mg protein/ml) in the presence of an NADPH-generating system. The apparent Km andVmax values were 29 ± 8 μM and 143 ± 15 pmol/min/mg protein for HL-4 (▵), 28 ± 2 μM and 281 ± 5 pmol/min/mg protein for HL-10 (□), and 435 ± 209 μM and 3040 ± 1170 pmol/min/mg protein for HL-3 (●), respectively. The kinetic parameters (means ± S.E.) were calculated from the fitted curves with the computer program KaleidaGraph.
Effects of P-450 Inhibitors and Anti-CYP Antibodies on Metabolite 3 Formation in Different Human Liver Microsomes.
Effects of P-450 inhibitors on metabolite 3 formation activities catalyzed by liver microsomes of HL-3 and HL-4 were determined at substrate concentrations of 10 and 30 μM troglitazone, respectively (Table 3). At a low substrate concentration, quercetin was effective in inhibiting metabolite 3 formation by human liver microsomes (sample HL-4). However, when 30 μM troglitazone was used, quercetin did not inhibit, even in sample HL-4. Ketoconazole inhibited metabolite 3 formation in both human liver microsomal samples at both substrate concentrations, but neither sulfaphenazole, fluvoxamine, nor quinidine inhibited metabolite 3 formation (Table 3). Inhibition of metabolite 3 formation was more extensive with a combination of quercetin and ketoconazole in human liver microsomal samples. In separate experiments, quercetin (10 μM) inhibited paclitaxel 6α-hydroxylation (at 10 μM substrate concentration) by ∼40% in human liver microsomal sample HL-4 (data not shown).
Effects of chemical inhibitors on quinone-type metabolite 3 formation from troglitazone catalyzed by human liver microsomes (samples HL-3 and HL-4)
Anti-CYP2C IgG strongly inhibited microsomal troglitazone oxidation activities at 10 μM in human liver microsomal sample HL-4, although the inhibitory effect of anti-CYP2C antibodies was less than of anti-CYP3A4 antibodies in microsomal sample HL-3 (Table4).
Effects of preimmune and anti-P-450 antibodies on quinone-type metabolite 3 formation of troglitazone catalyzed by human liver microsomes (samples HL-3 and HL-4)
Discussion
Troglitazone is a new oral hypoglycemic agent recently approved for use in type II diabetes mellitus. Troglitazone has a vitamin E-like structure (hydroxychroman moiety) and has been suggested to be nonenzymatically converted to a quinone-type metabolite by lipid peroxides or active oxygen (Fu et al., 1996). Although troglitazone is a potential inducer of CYP3A4 (Kaplan et al., 1998; Koup et al., 1998), it is reported not to be metabolized by CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4 (Physicians’ Desk Reference, 1999). Why has this information (Physicians’ Desk Reference, 1999) been reported regarding the roles of P-450 enzymes involved in the oxidation of troglitazone by human liver microsomes? It has been shown that there are variations in catalytic activities of recombinant P-450 enzymes expressed in different systems (Shaw et al., 1997; Yamazaki et al., 1997). In this study, metabolite 3 formation from troglitazone catalyzed by CYP3A4 was clearly shown (in a substrate-dependent manner) in the presence of NADPH or CuOOH, whereas microsomes of lymphoblastoid cells did not produce detectable amounts of metabolite 3. These findings may be related to the false negative reports (Physicians’ Desk Reference, 1999). It should be mentioned that the choice of recombinant P-450 enzyme systems is of great importance for drug metabolism research, and sensitivity may be the issue in this case. The reason why we focused the quinone-type metabolite formation is that metabolite 3 has been detected in human plasma after oral administration of troglitazone, and quinone-type metabolites are generally considered to be active intermediates in several drug-induced hepatic toxicities (Pumford and Halmes, 1997; Bort et al., 1999).
It has been reported that maximum plasma concentrations of troglitazone increase proportionally with increasing doses over the range of 200 to 600 mg/day (Physicians’ Desk Reference, 1999). After daily drug administration, steady-state plasma concentrations of troglitazone are reached within 3 to 5 days; the maximum plasma concentrations are 0.9 to 2.8 μg/ml (2.0–6.4 μM) in the steady state in normal volunteers (Physicians’ Desk Reference, 1999). In our preliminary experiments with microsomal protein of human liver and lymphoblastoid cells expressing CYP3A4, approximately half of an initial concentration of 5 μM troglitazone was not recovered in the absence or presence of an NADPH-generating system, similar to findings reported previously (Izumi et al., 1997b). We then chose the low- and high-substrate concentrations at 10 and 100 μM troglitazone, respectively, for further work.
Correlation analysis suggested that at least two or more P-450 enzymes in human liver microsomes might catalyze metabolite 3 formation at 10 μM troglitazone because no clear patterns were found. CYP3A4 would be a major catalyst at higher substrate concentrations. At 30 μM (an apparent single Km value in human liver microsomes), CYP3A4 was also a major enzyme involved in metabolite 3 formation because chemical inhibition was observed only with ketoconazole at this substrate concentration. Average levels of CYP3A4 in human livers have been determined to be ∼30% of total P-450 in Japanese and Caucasian samples examined; in some people CYP3A4 level accounts for >60% of total P-450, probably due to the induction by various chemical agents (Guengerich, 1995). The average content of CYP2C19 in human liver microsomes has been reported to be ∼1% of total P-450, and contents of CYP2C8 were much lower than those of CYP2C19 (Inoue et al., 1997). These findings support the idea that the contribution of P-450 enzymes in troglitazone oxidation reactions in human livers may be altered by using different human samples with compositions of various P-450 enzymes in the liver.
Using different human samples that contain varying levels of individual P-450 enzymes in the liver and recombinant human P-450 enzymes expressed in various systems, we obtained several lines of evidence to support the view that different human P-450 enzymes, particularly CYP2C8 and CYP3A4, contribute significantly to troglitazone oxidation to the quinone-type metabolite in humans and that the roles of these P-450 enzymes vary with the use of different human samples. The results obtained in this study can be summarized as follows. In human livers having relatively high contents of CYP2C and low CYP3A4 (a sample HL-4), anti-CYP2C and quercetin, an inhibitor against CYP2C8 (Rahman et al., 1994), suppressed this reaction significantly at low substrate concentrations (and recombinant CYP2C8 had a higherVmax/Km ratio than CYP3A4). However, the role of CYP3A4 is much greater in human samples that contain relatively high levels of CYP3A4, e.g., sample HL-3, and this reaction was inhibited by ketoconazole and anti-CYP3A4 even at low substrate concentrations. Apparently theKm component (∼30 μM) observed in human liver microsomes was higher that those of recombinant CYP2C enzymes (∼3 μM). No inhibitory effects of fluvoxamine, an inhibitor of both CYP1A2 and CYP2C19 (Jeppesen et al., 1996; Yamazaki et al., 1997); sulfaphenazole, an inhibitor of CYP2C9 (Mancy et al., 1996); or quinidine, an inhibitor of CYP2D6 (Otton et al., 1984), on metabolite 3 formation were observed, suggesting that CYP2C9 and CYP2C19 as well as CYP1A2 and CYP2D6 play only minor roles in metabolite 3 formation in human liver microsomes.
In conclusion, our results suggest that both CYP2C8 and CYP3A4 are major P-450 enzymes involved in the oxidation of troglitazone to a quinone-type metabolite in human livers. The roles of these two P-450s vary depending on the contents of CYP2C8 and CYP3A4 in livers. In general, CYP3A4 has the highest content of any P-450s in human liver (Shimada et al., 1994). Therefore, the CYP3A4-catalyzed quinone-type metabolite formation from troglitazone may have special significance in liver and other CYP3A4-rich sites. CYP2C8 may also play an essential contribution. This information about the roles of individual human P-450 enzymes in troglitazone oxidations is of relevance in evaluating hepatocellular injury in troglitazone treatment in humans. Studies of drug interaction caused by troglitazone via inhibition of P-450-supported drug metabolism is under investigation.
Acknowledgments
We thank Sankyo for providing troglitazone and its quinone-type metabolite 3 used in this study.
Footnotes
-
Send reprint requests to: Hiroshi Yamazaki, Ph.D., Division of Drug Metabolism, Faculty of Pharmaceutical Sciences, Kanazawa University, 13–1 Takara-machi, Kanazawa 920-0934, Japan. E-mail: yamazak{at}kenroku.kanazawa-u.ac.jp
-
Supported in part by grants from the Ministry of Education, Science, Sports, and Culture of Japan, and the Ministry of Health and Welfare of Japan.
- Abbreviations used are::
- P-450
- cytochrome P-450
- b5
- cytochromeb5
- CuOOH
- cumene hydroperoxide
- metabolite 3
- (±)-5-[4-[2-hydroxy-2-methyl-4-(3,5,6-trimethyl-1,4-benzoquinon-2-yl)butoxy]benzyl]-2,4-thiazolidinedione
- Received March 30, 1999.
- Accepted July 9, 1999.
- The American Society for Pharmacology and Experimental Therapeutics