Abstract
Some substrates of cytochrome P450 (CYP) 3A4, the most abundant CYP in the human liver responsible for the metabolism of many structurally diverse therapeutic agents, do not obey classical Michaelis-Menten kinetics and demonstrate homotropic and/or heterotropic cooperativity. The unusual kinetics and differential effects observed between substrates of this enzyme confound the prediction of drug clearance and drug-drug interactions from in vitro data. We have investigated the hypothesis that CYP3A4 may bind multiple molecules simultaneously using diazepam (DZ) and testosterone (TS). Both substrates showed sigmoidal kinetics in B-lymphoblastoid microsomes containing a recombinant human CYP3A4 and reductase. When analyzed in combination, TS activated the formation of 3-hydroxydiazepam (3HDZ) andN-desmethyldiazepam (NDZ) (maximal activation 374 and 205%, respectively). For 3HDZ, Vmax values remained constant with increasing TS, whereas the S50 and Hill values decreased, tending to make the data less sigmoidal. Similar trends were observed for the NDZ pathway. DZ inhibited the formation 6β-hydroxytestosterone (maximal inhibition, 45% of control), causing a decrease in Vmax but no significant change to the S50 and Hill values, suggesting that DZ may inhibit via a separate effector site. Multisite rate equation models have been derived to explore the analysis of such complex kinetic data and to allow accurate determination of the kinetic parameters for activation and inhibition. The data and models presented are consistent with proposals that CYP3A4 can bind and metabolize multiple substrate molecules simultaneously; they also provide a generic solution for the interpretation of the complex kinetic data derived from CYP3A4 substrates.
CYP3A4 is the most important human enzyme in the cytochrome P450 (CYP2) family due to its high relative abundance in the liver and its broad substrate specificity (Thummel and Wilkinson, 1998). In particular, CYP3A4 is known to play a critical role in several clinically relevant drug-drug interactions (Monahan et al., 1990; Baciewicz and Baciewicz, 1993; Olkkola et al., 1993). To be of optimal use, in vitro drug metabolism systems must accurately predict the metabolic fate and clearance of a drug in man and also the magnitude and likelihood of any clinically important interactions. In vitro studies with CYP3A4 have been confounded by some of the unusual properties of this enzyme. For example, some substrates do not obey classical Michaelis-Menten kinetics and demonstrate positive cooperativity and/or activation with the addition of a second compound, such as flavonoids or steroid hormones (Ueng et al., 1997; Korzekwa et al., 1998; Ludwig et al., 1999; Houston and Kenworthy, 2000). The active site of CYP3A4 is large enough to accommodate bulky molecules (e.g., erythromycin and cyclosporin), and it has been postulated that it may be capable of binding more than one molecule (Shou et al., 1994;Ueng et al., 1997; Korzekwa et al., 1998; Hosea et al., 2000). Several authors have suggested that CYP3A4 may be an allosteric protein, although the nature of the allosteric interaction is unclear (Lee et al., 1995). There are few examples of this phenomenon in vivo, but flavone-dependent activation of zoxazolamine metabolism has been observed in rats (Lasker et al., 1982), and quinidine has been shown to activate CYP3A4-mediated diclofenac metabolism in monkeys (Tang et al., 1999).
The interactions between substrates and inhibitors and/or activators of CYP3A4 are complex and difficult to predict given the current understanding of this enzyme; conflicting effects, including activation and varying levels of inhibition, may be observed depending on the substrate of the study (Kenworthy et al., 1999; Stresser et al., 2000;Wang et al., 2000). A greater understanding of the variability associated with CYP3A both in vitro and in vivo is required to fully understand the role of CYP3A in drug metabolism and thus improve the current capabilities for prediction with drugs metabolized by this enzyme. We have selected two substrates of CYP3A4, diazepam (DZ) and testosterone (TS), that are metabolized to 3-hydroxydiazepam (3HDZ) andN-desmethyldiazepam (NDZ) and 6β-hydroxytestosterone (6β-HTS), respectively. Both substrates have been found to exhibit sigmoidal kinetics in a variety of in vitro systems (Andersson et al., 1994; Lee et al., 1995; Shaw et al., 1997; Shou et al., 1999), and each represents a particular subclass of CYP3A4 substrates (Kenworthy et al., 1999).
We have investigated the metabolism of DZ and TS, both alone and in combination, using a heterologous expression system expressing both human CYP3A4 and CPR to characterize the role of multiple substrate binding sites in the interaction between these substrates. Multisite kinetic models (Segel, 1975) have been derived to describe the experimental data, and their utility in the interpretation of the potential interactions has been explored. Although the complex nature of the CYP3A4 interactions with substrates/modifiers necessitates the use of mechanistic models, it is important that these have a practical value in addition to a theoretical basis. The simultaneous monitoring of DZ and TS metabolism during coincubation illustrates kinetic trends not previously observed for CYP3A4, and our treatment of these data is unusual as it attempts to combine both the theoretical and practical requirements stated above.
Materials and Methods
Chemicals.
Microsomes from a human B-lymphoblastoid cell line engineered to express a recombinant human CYP3A4 and human CPR (increases the catalytic activity 3-fold compared with endogenous CPR only) were obtained from the GENTEST (Woburn, MA). DZ, 3HDZ, NDZ, TS, and 6β-HTS were obtained from Sigma Chemical Co. (Poole, Dorset, UK).14C-labeled diazepam (specific activity, 7271 KBq/mg) was obtained from Amersham Pharmacia Biotech UK, Ltd. (Little Chalfont, Buckinghamshire, UK). All other reagents were obtained from commercial sources and were of at least analytical grade.
DZ Kinetic Studies.
Incubations of DZ were carried out under linear conditions with respect to both incubation time (15 min) and protein concentration (0.5 mg/ml; equivalent to a CYP concentration of 23.5–38 pmol/ml). The kinetics of DZ metabolism were investigated (n = 9) over a substrate concentration range of 2.5 to 250 μM (triplicate incubations), this range being constrained by the assay sensitivity and the solubility limitations of the substrate. Experiments were carried out using a 0.2-ml reaction volume containing 23.5 pmol of CYP/ml in 0.1 M potassium phosphate buffer, pH 7.4. DZ was added to the incubations in dimethylformamide; the final solvent concentration did not exceed 1% of the total volume. All samples were preincubated at 37°C for 5 min in a shaking water bath, and each reaction was initiated by the addition of an NADPH-regenerating system (final concentration in each incubation: 1 mM NADP+, 7.5 mM isocitric acid, 15 mM magnesium chloride, and approximately 0.2 units of isocitric dehydrogenase). Each reaction was terminated after a 15-min incubation period by the addition of 20 μl of 10 M sodium hydroxide. An internal standard (prazepam; 70 μM) was added, and samples were extracted with 1 ml of carbonate buffer (100 mM; pH 10) and 5 ml of ethyl acetate; the organic layer was removed and evaporated to dryness under nitrogen and reconstituted in mobile phase. Aliquots (100 μl) were analyzed by high-pressure liquid chromatography with UV detection, according to the method of Reilly et al. (1990). Quantification of 3HDZ and NDZ concentrations was achieved by comparison of metabolite-to-internal standard peak height ratio with those of a calibration curve (range, 0.25–25 nmol). The on-column limit of quantification for 3HDZ and NDZ was 0.025 nmol, and the interassay coefficient of variation was less than 5% across the concentration range studied.
TS Kinetic Studies.
Incubations of TS were carried out under linear conditions with respect to both incubation time (15 min) and protein concentration (0.5 mg/ml; equivalent to a CYP concentration of 38 pmol/ml). The metabolism of TS was investigated over a substrate concentration range (n = 13) of 1.5 to 500 μM (triplicate incubations). Experiments were carried out using a 0.2-ml reaction volume containing 37.5 pmol of CYP/ml in 0.1 M potassium phosphate buffer, pH 7.4. TS was added to each incubation in methanol; the final solvent concentration did not exceed 1% of the total volume. All samples were preincubated at 37°C for 5 min in a shaking water bath, and each reaction was initiated by the addition of an NADPH-regenerating system (as above). Each reaction was terminated after a 15-min incubation period by the addition of 100 μl of acetonitrile. Precipitated proteins were sedimented by centrifugation (13,400g; 5 min), and aliquots (100 μl) of the supernatant were injected onto a high-pressure liquid chromatograph with a 15-cm × 3.9-mm i.d. Waters C18 Novapak column (Waters, Milford, MA) and a mobile phase consisting of 50% methanol/50% water at a flow rate of 1 ml/min. TS and its metabolites were quantified using UV detection at 254 nm. The retention times of 6β-HTS and TS were 5 and 29 min, respectively. Quantification of 6β-HTS concentrations was achieved by comparison of peak areas with those of a calibration curve (range, 0.05–7.5 nmol). The on-column limit of quantification for 6β-HTS was 0.0125 nmol, and the interassay coefficient of variation was less than 5% across the concentration range studied.
The Simultaneous Metabolism of DZ and TS by CYP3A4.
DZ and TS were incubated simultaneously (duplicate incubations) with all combinations of the following substrate concentrations: 0, 10, 25, 50, 100, and 250 μM for DZ (22–37 KBq/ml incubation mix) and 0, 5, 10, 25, 50, and 150 μM for TS. The DZ/TS molar ratios ranged from 0.07 to 50 (TS/DZ ratios from 0.04–15). DZ and TS were added to each incubation in methanol, and the final solvent concentration was 1% (v/v) in all incubations. The experiment was performed twice with the same batch of microsomes, using the appropriate analytical methodology for each substrate. When coincubated with TS, DZ and its metabolites were analyzed by radiochemical detection without addition of internal standard as detailed above; 3HDZ and NDZ concentrations were quantified from the percentage of the total radioactivity in each chromatogram. TS and its metabolites were analyzed by UV detection as detailed above. DZ and its metabolites did not interfere with the assay.
Analysis of Kinetic Data.
Kinetic data for each substrate alone and at each inhibitor concentration were analyzed by weighted nonlinear regression (WinNonlin; Scientific Consulting, Inc., Apex, NC ) using a sigmoidalVmax model equivalent to the Hill equation and a weighting factor of 1/y. The goodness of fit was determined by visual inspection of residual patterns, reduction in the residual sums of squares, the precision of the parameter estimates, and a reduction in the value of the fitting criteria (χ2 and Akaike information criterion values). The Hill equation (eq. 1) was used to determine the kinetic parametersVmax, S50, and the Hill coefficient (n):
Data were also modeled to evaluate the proposed hypothesis that CYP3A4 has multiple binding sites capable of binding more than one substrate simultaneously. Rate equation models (Fig.1) were adapted from those described bySegel (1975), assuming rapid equilibrium between the formation of the various enzyme species, according to the changes observed for each substrate combination. Each complete data set (n = 24) for DZ or TS in the presence of activator or inhibitor was fitted to equations derived from these models by linear regression using Grafit (Erithacus Ltd, Middlesex, UK). Fits from several models were tested for each data set; the models with the least number of kinetic parameters and those that were consistent with the model used for the other substrate were selected. Goodness of fit was determined by comparison of statistical parameters (χ2 and the Akaike information criterion values) between the models and a reduction in the standard errors of the parameter estimates.
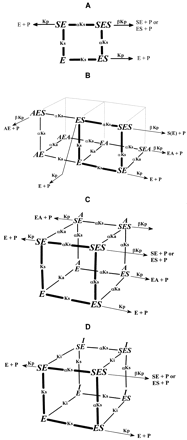
Multisite kinetic equilibria models.
A, two-site model for an enzyme with two identical binding sites. The corresponding equation is shown in eq. 3. B, two-site model for an enzyme in which two substrates interact competitively with two binding sites (shown in three-dimensional format for comparison with model C and D). The corresponding equation is shown in eq. 4. C, three-site model with heteroactivation for an enzyme in which two substrates interact with two binding sites and an activator acts at a third site. The corresponding equation is shown in eq. 5. D, three-site model with inhibition for an enzyme in which two substrates interact with two binding sites and an inhibitor acts at a third site. The corresponding equation is shown in eq. 6.
Multisite Kinetic Equilibria Models.
The models used were adopted from Segel (1975), and the reader is referred to this textbook for the assumptions made in his approach. Although these models may not be fully complete in a theoretical sense, they do allow multiple sets of data to be fitted to a single equation. The relationship between the four models used is outlined below. The simplest scheme (model A, two-site model; Fig. 1A) describes an allosteric enzyme that can simultaneously bind two molecules of the same substrate at identical binding sites. Product is formed from the single-substrate bound entities, SE and ES, and from the two-substrate bound entity, SES. If the reaction shows cooperativity, the binding affinity (Ks) changes by the factor α; if α < 1, the binding affinity for the second substrate molecule is increased, enhancing the overall product formation rate. Alternatively, two occupied sites may interact to change the effective catalytic rate constant (Kp) by the factor β in the two-substrate bound complex. If β > 1, the overall rate of the reaction is increased, and if β < 1, the overall rate is decreased. The velocity equation for this scheme is given in eq. 3. This model can be used to describe data from substrates showing autoactivation or sigmoidicity and substrate inhibition (Houston and Kenworthy, 2000).
Results
Kinetics of DZ and TS Metabolism in B-Lymphoblastoid Microsomes Expressing CYP3A4.
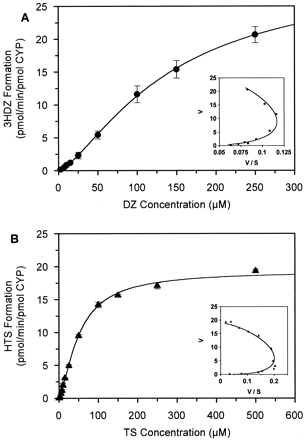
The formation of 3HDZ from DZ in GENTEST B-lymphoblastoid microsomes expressing CYP3A4 exhibits a sigmoidal kinetic profile, as characterized by a curved Eadie-Hofstee plot (Fig.2A). Formation of the minor metabolite, NDZ also showed a sigmoidal profile (Fig. 4); however, the levels were difficult to quantify at low substrate concentrations due to the low metabolic turnover of this pathway, so a full kinetic characterization is not shown. No other metabolic products were detected under the incubation conditions used in this in vitro system. The formation of 6β-HTS from TS in the same expression system also demonstrated sigmoidal kinetics (Fig. 2B). 6β-HTS was the main metabolite detected; small amounts of 15β- and 2β-hydroxytestosterone were also detected but could not be quantified over a wide enough substrate concentration range to characterize the kinetic profile.
Kinetic analysis of 3HDZ (A) formation from DZ and 6β-HTS (B) formation from TS in B-lymphoblastoid microsomes expressing CYP3A4 and CPR.
Eadie-Hofstee plots are shown as insets on each graph. Each data point represents the mean of triplicate determinations and is shown with standard error bars.
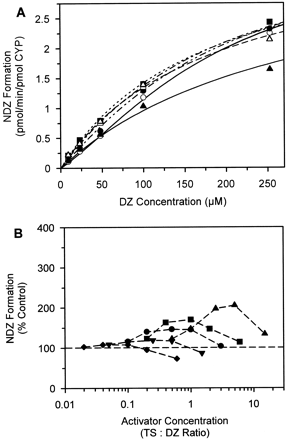
The effects of TS on NDZ formation.
A, kinetic plots of NDZ formation at TS concentrations of 0 μM (○), 5 μM (●), 10 μM (■), 25 μM (▪), 50 μM (▵), and 150 μM (▴); the solid and dashed lines represent the combined fit to eq. 4of the DZ data in the presence of 0, 5, 10, 25, 50, and 150 μM TS. B, activation of NDZ formation with increasing TS/DZ ratios at DZ concentrations of 10 μM (▴), 25 μM (▪), 50 μM (●), 100 μM (▾), and 250 μM (♦). Data points represent the mean of duplicate determinations.
The data for both DZ and TS metabolites could be well described by either the Hill equation (eq. 1) or a two-site rate equation model (eq.3), and the resulting kinetic parameters are given in Table1. The Vmaxvalues for the 3HDZ pathway was approximately 10-fold greater than that of the NDZ pathway, whereas the S50 values were similar for both pathways (ca. 150 μM). The differences observed in CLmax are mainly a consequence of the differences in the Vmax values. The extent of cooperativity was less marked for the NDZ pathway, as indicated by the lower n (Hill equation) and higher α (two-site equation) values. The S50 andKs value for 6β-HTS formation was approximately 3-fold lower than that for 3HDZ formation, indicating that autoactivation occurred at a lower substrate concentration, even though the extent of sigmoidicity (n value) was the same for both substrates.
Kinetic analysis of diazepam 3-hydroxylation and N-demethylation and testosterone 6β-hydroxylation in B-lymphoblastoid microsomes expressing CYP3A4 and CPR
The Simultaneous Metabolism of DZ and TS by CYP3A4.
The formation rates of both 3HDZ and NDZ from DZ were activated in the presence of increasing TS concentrations (Figs. 3A and 4A); this effect was most pronounced at low DZ concentrations. Activation of the 3HDZ pathway was greatest (374% of control values; Fig. 3B) at high TS/DZ ratios (>1), with little activation below TS/DZ ratios of 0.1. Analysis with the Hill equation at each inhibitor concentration (data not shown) was used to determine general trends in the effects of TS on DZ and to aid the choice of the most suitable multisite model to describe the data for both DZ and TS. The Vmax values for 3HDZ remained constant with increasing TS concentration, whereas the S50 decreased by approximately 30% (p < 0.05) and n values decreased from 1.4 to 1.2 (p < 0.005) over the concentration range studied. The change in sigmoidicity is also apparent in a decrease in the curvature of the Eadie-Hofstee plot (not shown) with increasing TS concentration. For the minor pathway NDZ, similar trends were observed (Fig. 4A) but of a smaller magnitude, the maximal activation being 205% (Fig. 4B). TheVmax decreased at high DZ and TS concentrations, suggesting that both activation and inhibition may be occurring for this pathway.
The effects of TS on 3HDZ formation.
A, kinetic plots of 3HDZ formation at TS concentrations of 0 μM (○), 5 μM (●), 10 μM (■), 25 μM (▪), 50 μM (▵), and 150 μM (▴); the solid and dashed lines represent the combined fit to eq. 5 of the DZ data in the presence of 0, 5, 10, 25, and 50 μM TS. B, activation of 3HDZ formation with increasing TS/DZ ratios at DZ concentrations of 10 μM (▴), 25 μM (▪), 50 μM (●), 100 μM (▿), and 250 μM (♦). Data points represent the mean of duplicate determinations.
The rate of 6β-HTS formation was inhibited in the presence of increasing DZ concentrations (Fig. 5A). The extent of inhibition was similar for all TS concentrations studied, the maximum inhibition being approximately 45% of control values at the highest DZ concentration studied (Fig. 5B). Hill analysis showed that Vmax decreased with increasing DZ concentration (p < 0.005) from 19 pmol/min/pmol of CYP (no DZ) to 10 pmol/min/pmol of CYP (250 μM DZ), whereas the S50 and Hill coefficient showed no significant change from 51 and 1.4 μM, respectively. The change toVmax while the S50value remains constant is also demonstrated by parallel fits when the data are transformed on an Eadie-Hofstee plot (not shown), and the comparable curvature indicates that the n value does not change with increasing inhibitor concentration.
The effects of DZ on 6β-HTS formation.
A, kinetic plots of 6β-HTS formation at DZ concentrations of 0 μM (○), 10 μM (●), 25 μM (■), 50 μM (▪), 100 μM (▵), and 250 μM (▴); the solid and dashed lines represent the combined fit to eq. 6 of the TS data in the presence of 0, 10, 25, 50, 100, and 250 μM TS. B, activation of 6β-HTS formation with increasing DZ/TS ratios at TS concentrations of 5 μM (▴), 10 μM (▪), 25 μM (●), 50 μM (▾), and 150 μM (♦). Data points represent the mean of duplicate determinations.
The data presented in Figs. 3 to 5 have been simultaneously fitted to the equations derived from the multisite models shown in Fig. 1, B to D, respectively. The kinetic parameters (Table2) for each substrate are consistent between models. The Ks for TS binding is in good agreement with the Ka for the activation of DZ metabolism. The Ki for DZ inhibition of TS is intermediate to the Ksvalues of the two DZ sites. The α values for the two DZ metabolites are different, reflecting that the 3HDZ pathway shows greater positive cooperativity in this enzyme system. Identical α values were obtained for both the 3HDZ and the 6β-HTS pathways, indicating that the extent of autoactivation is the same for the formation of these metabolites from the two substrates. The extent of 3HDZ activation observed at all TS concentrations and the unchanged S50 andn values for 6β-HTS when inhibited by DZ suggest that the effector may be acting at another distinct site and does not displace the substrate molecules from the active site. However, the 3HDZ and NDZ data may also be described by a model (Fig. 1B.) in which the effector competes with the substrate for binding. In this model, activation results from an increase in the Kp for DZ metabolism of approximately 2-fold in the presence of TS.
Kinetic analysis of the inhibition effects when diazepam and testosterone are incubated simultaneously with B-lymphoblastoid microsomes expressing CYP3A4 and CPR
Discussion
The cooperative binding effects associated with substrates of CYP3A4 are well documented, and it has become widely accepted that such events may be due to the binding of multiple molecules to the enzyme, either within the active site (Shou et al., 1994; Korzekwa et al., 1998; Shou et al., 1999; Domanski et al., 2000) or at separate, distant locations on the enzyme (Schwab et al., 1988; Ueng et al., 1997). The nature of the interactions between substrates, effectors, and enzyme is likely to vary depending on the molecules under investigation, as well as with other variables including CPR, cytochromeb5, and buffer components (Yamazaki et al., 1996; Maenpaa et al., 1998; Schrag and Wienkers, 2001). To overcome the difficulties of interpreting atypical in vitro data with CYP3A4, several groups have proposed that a two-site model may be appropriate (Ueng et al., 1997; Korzekwa et al., 1998; Shou et al., 1999; Houston and Kenworthy, 2000). This is a more useful approach for the analysis of sigmoidal data than the Hill equation in which the parameters bear no direct relation to those of the Michaelis-Menten equation. In the investigations described here, we have demonstrated the utility of multisite models for the kinetic analysis of data from two substrates that are metabolized simultaneously by CYP3A4 where substrate activation, heteroactivation, and inhibition are observed.
The formation of 3HDZ and NDZ from DZ and the formation of 6β-HTS from TS showed sigmoidal kinetics, which is in agreement with earlier findings using other expression systems and human liver microsomes (Andersson et al., 1994; Shaw et al., 1997; Shou et al., 1999). Also, there have been numerous reports of CYP3A4 autoactivation via steroid hormones (Schwab et al., 1988; Lee et al., 1995; Ueng et al., 1997;Harlow and Halpert, 1998; Domanski et al., 1998).
When DZ and TS are incubated simultaneously, the formation of 3HDZ was increased at all TS concentrations, and no inhibition was observed. The two potential models (B and C) can be used to describe the heteroactivation observed with DZ. In the latter case, TS may act at a distinct effector site, where it induces a conformational change in the enzyme and alters the overall velocity of the SES complex. Alternatively, it may cause a change in the effective catalytic rate constant when one substrate and one effector molecule are bound. Both models adequately describe the experimental data for 3HDZ and generate similar kinetic parameters and curve fits, and they cannot be distinguished simply by kinetic measurements. Model C gives the best fit for the NDZ pathway since some inhibition of this pathway is evident at higher substrate concentrations, indicating competition. When this model for the 3HDZ pathway is fitted to the data, theKp interaction factor β is 2. This is consistent with the alternative three-site model D in which theKp values of the two DZ sites are unaffected in the presence of TS, but product is also generated from the SESA complex. The latter model has the least number of kinetic parameters and generates a better fit to the 3HDZ data.
The metabolism of TS is inhibited by DZ and could result from displacement of TS from the active site by DZ. However, the extent of cooperativity observed for TS does not significantly change with increasing DZ concentration. The inhibition effects are also manifested as a change in the Vmax, with no significant change to the S50 value, as would be the case in competitive inhibition. Additionally, the IC50 value does not change with increasing substrate concentration. These factors strongly suggest that DZ may be causing inhibition at a site separate to that for TS metabolism. The S50 value for DZ is 3-fold higher than that for TS metabolism. Therefore, by analogy with the typical Michaelis-Menten system, it would be expected that TS would be likely to cause inhibition of DZ given the greater affinity for the enzyme. However, the observed results show the opposite to be true, with TS causing extensive activation of DZ metabolism and DZ causing inhibition of TS metabolism. The three-site model fitted well to the experimental data for 6β-HTS formation. The data cannot be described by the two-site model B because, contrary to this model, high concentrations of DZ do not eliminate the sigmoidicity of 6β-HTS formation.
The three-site models (C and D) used for the major metabolic pathways of DZ and TS when both are metabolized by CYP3A4 are consistent with each other; both have two substrate binding sites and a distinct effector site. Although other models cannot be ruled out, for the purpose of describing the data sets obtained in these experiments, this was the simplest model that described the data well. In those models in which there are numerous interaction factors, the parameters cannot necessarily be defined accurately due to the potential for multiple solutions when performing a regression analysis.
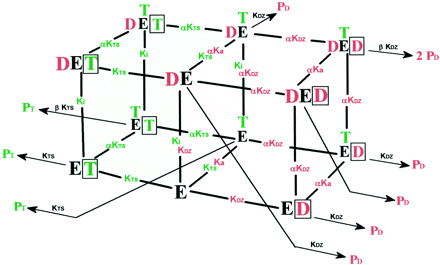
The models for DZ and TS can be combined to evaluate the minimum number of distinct sites needed to describe the effects observed with both substrates (Fig. 6). Individual sites must be in close proximity to the active oxygen if they are catalytically active. The simplest combined scheme has three sites—one that binds DZ, one that binds TS, and one that is capable of binding either DZ or TS. In the combined model, the boxed site is not catalytically active for 6β-HTS formation in the presence of DZ and appears to have only a regulatory function, suggesting that DZ may obscure TS from the active oxygen. Kinetic parameters and constants are in good agreement when they are on the shared face of the combined model. The binding constant for TS is similar to the binding constant for the activation of DZ metabolism (137 and 148 μM, respectively). The Ki for the inhibition of TS metabolism (186 μM) is intermediate to the binding constant for DZ (KDZ) with positive cooperativity (between 640 and 64 μM). The formation of 3HDZ is activated to a greater extent than NDZ by TS, suggesting that in the presence of TS this pathway is favored. TS may alter the conformation of the active site allowing DZ easier access to the active oxygen, while being in a position less favorable for metabolism itself. This scenario can explain mutual activation and inhibition observed when DZ and TS are incubated simultaneously.
Combined kinetic equilibria model describing the simultaneous metabolism of DZ and TS and the mutual activation/inhibition.
Model 1C and 1D are represented as red and green cubes, respectively. Substrate binding to individual sites (D and T), kinetic constants (KDZ, KTS,Ka, Ki, and α), and product formation (PD and PT) are represented in red and green lettering for DZ and TS, respectively.
The potential for binding to more than two sites on CYP3A4 has also been alluded to by Shou et al. (1994) and more recently by Domanski et al. (2000) and Hosea et al. (2000). It has been proposed that the site for metabolism and activation by an effector may be distinct since two cooperative substrates have not been shown to cause mutual inhibition. Studies with site-directed mutants of CYP3A4 also support the hypothesis that both substrate and effector sites are closely linked and may be involved in substrate and/or effector binding, depending on the molecule of study (Harlow and Halpert, 1998; Domanski et al., 2000). It is of interest that Hosea et al. (2000), in their CYP3A4 binding studies using non metabolized peptides, have also found the need for a three-site model.
Although the models presented here may be viewed as more complicated that others reported earlier (Ueng et al., 1997; Korzekwa et al., 1998;Shou et al., 1999), it is quite likely that although only two or three molecules may be able to bind to the enzyme at any one time, there may be many discrete sites that can accommodate each particular substrate conformation. This may be due to the large, relatively indiscriminate nature of the active site of CYP3A4 and can also account for the ability of CYP3A4 to metabolize substrates in several different locations. The exact binding conformations depend on the combination of substrates and effectors being studied and their relative concentration. These “pockets” within the active site probably overlap, producing a multitude of potential substrate combinations, hence generating the plethora of effects that can be observed when two or more drugs interact with CYP3A4. The possibility that the effector may also be interacting at other independent sites either on CYP or with other accessory proteins, such as CPR, cannot be ruled out.
In conclusion, the analysis of the complicated interaction between DZ and TS by the application of a common enzyme model is made easier by the availability of two simultaneously generated data sets. However, due to the complexity of the data, there may not be a unique solution for such data sets. Comparison of the interactions between multiple CYP3A4 substrates enhances our understanding of the substrate binding patterns for this isoform. The effects of one substrate on the metabolism of another appears to be dependent on the substrate of use (Kenworthy et al., 1999; Wang et al., 2000). The combination of two small molecules, both showing sigmoidal kinetics when studied in isolation, is likely to be one of the more complex scenarios encountered with this enzyme. The application of different but similar models demonstrates that the kinetic parameter estimates (Ki or Ka) are comparable regardless of the model chosen, indicating the robust nature of kinetic equilibria models as a tool for the in-depth study of drug-drug interactions associated with CYP3A4 in vitro.
Footnotes
-
↵1 Present address: Department of Mechanism and Extrapolation Technologies, GlaxoSmithKline, The Frythe, Welwyn, Herts, AL6 9A, UK.
-
K.E.K. was financially supported by a SmithKline Beecham studentship. A portion of this study was presented at the meeting of the British Pharmacological Society, December 10–12, 1997, Harrogate, UK and appeared in abstract form in Br J Clin Pharmacol45:520P–521P (1998).
- Abbreviations used are::
- CYP
- cytochrome P450
- DZ
- diazepam
- TS
- testosterone
- 3HDZ
- 3-hydroxydiazepam
- NDZ
- N-desmethyldiazepam
- 6β-HTS
- 6β-hydroxytestosterone
- CPR
- cytochrome P450 reductase
- CLmax
- clearance at maximal activation
- Received May 25, 2001.
- Accepted September 18, 2001.
- The American Society for Pharmacology and Experimental Therapeutics