Abstract
Recently, a chimeric mouse line in which the liver could be replaced by more than 80% with human hepatocytes was established in Japan. Because the chimeric mouse produces human albumin (hAlb), replacement by human hepatocytes could be estimated by the hAlb concentration in the blood of chimeric mice. In this study, we investigated human major cytochrome P450 (P450) in the livers of chimeric mice by mRNA, protein, and enzyme activity using real-time polymerase chain reaction, Western blot analysis, and high-performance liquid chromatography, respectively. Chimeric mice with humanized liver generated using hepatocytes from a Japanese and white donor were used. Human P450 mRNAs were expressed in the liver of chimeric mice, and major human P450 proteins such as CYP1A2, CYP2C9, and CYP3A4 were detected. The expression of P450 mRNA and protein was correlated with the hAlb concentration in the blood. The enzyme activities such as diclofenac 4′-hydroxylase activity, dexamethasone 6-hydroxylase activity, and coumarin 7-hydroxylase activity, activities that are specific to human P450 but not to murine P450, were increased in a hAlb concentration-dependent manner. The chimeric mice with nearly 90% replacement by human hepatocytes demonstrated almost the same protein contents of human P450s and drug-metabolizing enzyme activity as those of the donor. It was confirmed that genomic DNA from the livers of the chimeric mice and that from the liver of the donor exhibited the same genotype. In conclusion, the chimeric mice exhibited a similarly efficient capacity of drug metabolism as humans, suggesting that they could be a useful animal model for drug development.
Recently, efforts have been made toward optimizing drug treatment by taking into account individual differences in drug metabolism, which are regarded as major determinants of the interindividual variability in pharmacokinetics, clinical efficacy, and toxicity of drugs (Lu, 1998). Cytochrome P450 (P450) plays a central role in drug metabolism in human liver. One of the isoforms, CYP3A4, is predominantly expressed in human liver and has been reported to be responsible for the metabolism of more than 50% of the clinical drugs (Pelkonen et al., 1998). In addition, several human P450s have been shown to be polymorphic due to single nucleotide polymorphisms, gene deletions, and gene duplications (Ingelman-Sundberg, 2002). These polymorphisms can also affect the pharmacokinetics, safety, and efficacy of the drugs. Detailed analyses of drug metabolism catalyzed by human P450s are very important in drug development.
Various approaches have been used to evaluate the metabolism of drug candidates in humans at early preclinical stages. In vitro approaches using human liver microsomes (HLM), recombinant human P450 microsomes, human hepatocytes, and cDNA-expressing cells have been reported. These in vitro models have various advantages and limitations, as have been described previously (Gomez-Lechon et al., 2003; Rodrigues and Rushmore, 2003). Briefly, HLM are commercially available and can be stored for years with minimal or no loss of P450 enzyme activities, but it is impossible to use them for P450 induction studies. Although human hepatocytes retain all the drug-metabolizing enzymes, the enzyme activities are decreased during culture. These human-derived samples are limited in supply. In addition, although in vivo approaches using experimental animals are able to clarify the pharmacokinetics of drugs in the whole body, human and animal P450s are known to often be functionally distinct.
Several attempts have been made to develop bioartificial livers. Watanabe et al. (2003) established the immortalized human hepatic stellate scavenger cells that could be used as a bioartificial liver. Although this model was shown to express CYP3A4 and CYP2C9 proteins, the enzyme activities in these cells were not clarified. It was reported that the livers in urokinase-type plasminogen activator (uPA)/recombinant activation gene-2 mice could be repopulated with approximately 15% normal human hepatocytes isolated from adult liver and that these mice could be inoculated with human hepatitis B virus (Dandri et al., 2001). Mercer et al. (2001) have reported the generation of chimeric uPA+/+/SCID mice with human cells occupying a portion of the cross-sectional liver area, and these mice could be successfully infected with human hepatitis C virus. These studies were valuable for the development of new treatments against hepatitis virus and for the progress of regenerative medicine; however, the replacement of mouse liver with human hepatocytes was low and insufficient in these reports (Dandri et al., 2001; Mercer et al., 2001). Recently, chimeric mice in which the livers could be replaced by more than 80% with human hepatocytes were established by Tateno et al. (2004). We speculated that such mice could be used for predicting drug metabolism in humans, since the livers of the chimeric mice were almost completely replaced with human hepatocytes. In the present study, we investigated the expression of human P450s in the livers of such mice to determine whether they can be used as a new tool for studies in drug development.
Materials and Methods
Materials. All primers shown in Table 1 were commercially synthesized at Hokkaido System Sciences (Sapporo, Japan). Polyclonal rabbit anti-human CYP1A2 antibody, polyclonal rabbit anti-human CYP2A6 antibody, and polyclonal rabbit anti-human CYP2C8 antibody were purchased from Nosan (Yokohama, Japan). Polyclonal rabbit anti-human CYP2C9 antibody and human CYP2D6, CYP3A4, and CYP3A5 Western blotting kits were obtained from BD Gentest (Worburn, MA). Recombinant human CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2D6, and CYP3A4 expressed in baculovirus-infected insect cells were also obtained from BD Gentest. Recombinant human CYP3A5 was obtained from Invitrogen (Carlsbad, CA). Debrisoquine, diclofenac, and paclitaxel were purchased from Sigma-Aldrich (St. Louis, MO). Coumarin, 7-hydroxycoumarin, and dexamethasone were purchased from Wako Pure Chemical Industries (Osaka, Japan). S-Mephenytoin was obtained from Toronto Research Chemicals (Toronto, ON, Canada). (±)-4′-Hydroxydebrisoquine and (±)-4′-hydroxymephenytoin were purchased from Ultrafine Chemicals (Manchester, UK). Pooled HLM, 4′-hydroxydiclofenac, and 6α-hydroxypaclitaxel were purchased from BD Gentest. Nicotinamide adenine dinucleotide phosphate (oxidized form, NADP+) and glucose-6-phosphate dehydrogenase were purchased from Oriental Yeast (Tokyo, Japan). Nafamostat mesilate was kindly provided by Torii Pharmaceutical (Tokyo, Japan). All other chemicals and solvents were of the highest grade commercially available.
Sequence of the primers used in the present study
Generation of the Chimeric Mice with Humanized Liver. The present study was approved by the Ethics Committees of Kanazawa University and the Hiroshima Prefectural Institute of Industrial Science and Technology Ethics Board. The human liver sample from donor A (Japanese, male, 12 years old) was obtained at autopsy after receiving written informed assent. The cryopre-served human hepatocytes from donor B (white, male, 9 months old) were purchased from In Vitro Technologies (Baltimore, MD). The chimeric mice with humanized liver were generated by the method described previously (Tateno et al., 2004). Briefly, uPA+/+/SCID mice at 20 to 30 days after birth were injected with human hepatocytes through a small left-flank incision into the inferior splenic pole. When necessary, the chimeric mice were treated intraperitoneally with nafamostat mesilate. The concentration of human albumin (hAlb) in the blood of the chimeric mice and the replacement index (RI; the rate of the replacement from mice to humans) were measured using enzyme-linked immunosorbent assay and anti-human specific cytokeratin 8 and 18 antibody, respectively. There was a good correlation between the hAlb concentration and the RI (Tateno et al., 2004). The male chimeric mice used in this study were 11 to 14 weeks old (Table 2). The uPA+/+/SCID mice, uPA+/-/SCID mice, and uPA-/-/SCID mice were obtained as previously reported (Tateno et al., 2004).
Chimeric mice used in the present study
Hepatic RNA Extraction and Real-Time Reverse Transcription-PCR. Human P450 mRNA was quantified by real-time reverse transcription-PCR. Total hepatic RNA was extracted using ISOGEN (Nippon Gene, Tokyo, Japan), and cDNAs were synthesized as described previously (Iwanari et al., 2002). The sequences of primers that were specific to each human P450 are shown in Table 1. PCR was performed using the Smart Cycler (Cepheid, Sunnyvale, CA) with Smart Cycler software (version 1.2b). The PCR conditions were as follows. After an initial denaturation at 95°C for 30 sec, the amplification was performed by denaturation at 94°C for 4 s and annealing and extension at 64°C for 20 s for 45 cycles. Amplified products were monitored directly by measuring the increase of the dye intensity of the SYBR Green I (Molecular Probes, Eugene, OR) that binds to double-strand DNA amplified by PCR. The copy number of mRNA in the cDNA samples was calculated using standard amplification curves. It was confirmed that the primer for human P450s used in this study did not cross-react with murine P450 mRNA. We could not obtain total RNA from chimeric mouse 3.
Genomic DNA Preparation from Livers. Genomic DNA from the liver of donor A and that of the chimeric mice were extracted with phenol/chloroform followed by ethanol precipitation (Brilliant et al., 1991). The genotyping of CYP2A6*4, CYP2C19*2, CYP2C19*3, and CYP3A5*3 was performed as described previously (de Morais et al., 1994; Fukuen et al., 2002; Nakajima et al., 2004).
Liver Microsomes. Liver microsomes were prepared as described previously (Yamazaki et al., 1999a) and stored at -80°C until analysis. The protein concentration was determined using Bradford protein assay reagent (Bio-Rad, Hercules, CA) with bovine gamma globulin as the standard. We could not obtain liver microsomes from donor B.
Immunoblot Analysis of Human P450 Isoforms. SDS-polyacrylamide gel electrophoresis and immunoblot analysis of human CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2D6, CYP3A4, and CYP3A5 were performed according to Laemmli (1970), with slight modifications. The liver microsomes (5-20 μg) were separated on 7.5% polyacrylamide gel and transferred electrophoretically to a polyvinylidene difluoride membrane. Recombinant human P450s were also applied as the standards. Biotinylated anti-rabbit or mouse IgG and a VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA) were used for diaminobenzidine staining. It was confirmed that the human P450 antibodies in this experimental condition did not cross-react with the autologous murine P450 proteins.
Enzyme Assays. The typical incubation mixtures (total volume, 0.20 ml) consisted of microsomes in 100 mM potassium phosphate buffer (pH 7.4) containing an NADPH-generating system (0.5 mM NADP+, 5 mM glucose 6-phosphate, 5 mM MgCl2, and 1 U/ml glucose-6-phosphate dehydrogenase) and a substrate. Coumarin 7-hydroxylase activity (COH) was measured as described previously (Yamazaki et al., 1999b). Briefly, the concentrations of microsomes and coumarin were 0.1 mg/ml and 1 μM, respectively. The reaction mixture was incubated for 3 min at 37°C. The product formation was determined using high-performance liquid chromatography (HPLC) with a C18 5-μm analytical column (4.6 × 150 mm). Paclitaxel 6α-hydroxylase activity (PTXOH) was determined by the method of Willey et al. (1993), with slight modifications. The concentrations of microsomes and paclitaxel were 0.2 mg/ml and 20 μM, respectively. The reaction mixture was incubated for 10 min at 37°C. The mobile phase was acetonitrile/10 mM ammonium acetate = 40:60 (v/v). The product formation was determined using HPLC with a C18 5-μm analytical column (4.6 × 150 mm). The eluent was monitored at 227 nm with a noise-base clean Uni-3 (Union, Gunma, Japan). Diclofenac 4′-hydroxylase activity (DICOH) was determined by the method of Kobayashi et al. (2000), with slight modifications. The concentrations of microsomes and diclofenac were 0.2 mg/ml and 30 μM, respectively. The reaction mixture was incubated for 30 min at 37°C. The mobile phase was 22% acetonitrile in 50 mM phosphate buffer (pH 7.4). The product formation was determined using HPLC with a C18 5-μm analytical column (4.6 × 150 mm). S-Mephenytoin 4′-hydroxylase activity (MPOH) was measured as described previously (Chiba et al., 1993), with slight modifications. The concentrations of microsomes and S-mephenytoin were 0.2 mg/ml and 200 μM, respectively. The reaction mixture was incubated for 30 min at 37°C. The product formation was determined using HPLC with a C18 5-μm analytical column (4.6 × 150 mm). The eluent was monitored at 204 nm with a noise-base clean Uni-3. The mobile phase was 18% acetonitrile in 50 mM potassium dihydrogen phosphate. Debrisoquine 4′-hydroxylase activity (DBOH) was determined by the method of Nakajima et al. (2002b). The concentrations of microsomes and debrisoquine were 0.2 mg/ml and 5 μM, respectively. The eluent was monitored fluorometrically (excitation, 219 nm; emission, 286 nm) with a noise-base clean Uni-3. Dexamethasone 6-hydroxylase activity (DEXOH) was performed according to the method of Tomlinson et al. (1997), with slight modifications. The concentrations of microsomes and dexamethasone were 0.2 mg/ml and 100 μM, respectively. The reaction mixture was incubated for 30 min at 37°C. The product formation was determined using HPLC with a C8 5-μm analytical column (4.6 × 150 mm). The mobile phase was 22% acetonitrile/0.018% formic acid = 18:82 (v/v). The eluent was monitored at 243 nm with a Uni-3. DEXOH was quantified using a standard curve of dexamethasone because we could not obtain authentic 6-hydroxydexamethasone. The retention time of 6-hydroxydexamethasone was confirmed using the incubation product of recombinant CYP3A4 and dexamethasone. The final concentration of the solvent in the incubation mixture was <1%.
Results
Chimeric Mice Used in the Present Study. Five chimeric mice generated using hepatocytes from donor A and seven chimeric mice generated using those from donor B were used in the present study. The hAlb concentration and RI are shown in Table 2. We confirmed that the expression of all hepatic mRNA and all enzyme activities in the chimeric mice were not affected by the administration of nafamostat mesilate (data not shown).
Expression of Human CYP2C9 in Chimeric Mice. The expression of human CYP2C9 mRNA, the expression of human CYP2C9 protein, and DICOH in the chimeric mice were increased in a hAlb concentration-dependent manner (Fig. 1). The r values between mRNA, protein, or DICOH and a hAlb concentration in the donor A and donor B chimeric mice were 0.92 and 0.72, 0.97 and 0.95, and 0.98 and 0.93, respectively. In chimeric mouse 5 with hepatocytes from donor A, both the expression of human CYP2C9 mRNA and the expression of human CYP2C9 protein were almost the same as those in donor A. DICOH was mainly catalyzed by human CYP2C9 but not by murine Cyp2c. DICOH in the pooled HLM (1.80 nmol/mg of protein/min) was approximately 20-fold higher than that in uPA+/-/SCID mice (0.09 nmol/mg protein/min) and uPA-/-/SCID mice (0.09 nmol/mg protein/min). DICOH in microsomes from donor A (0.74 nmol/mg protein/min) was 41% compared with that in the pooled HLM.
Human CYP2C9 expression in the chimeric mice. Relative expression levels of human CYP2C9 mRNA (A), the expression of human CYP2C9 protein (B), and DICOH (C) were determined as described under Materials and Methods. C, DICOH catalyzed by CYP2C9 measured at 30 μM diclofenac. Each column represents the mean of duplicate determinations except in uPA+/-/SCID mice and uPA-/-/SCID mice. The columns of uPA+/-/SCID mice and uPA-/-/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
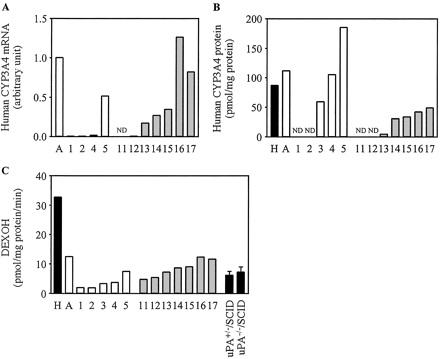
Expression of Human CYP3A4 in Chimeric Mice. The expression of human CYP3A4 mRNA, the expression of human CYP3A4 protein, and DEXOH were increased in a hAlb concentration-dependent manner (Fig. 2). The r values between mRNA, protein, or DEXOH and a hAlb concentration in the donor A and B chimeric mice were 0.91 and 0.69, 0.96 and 0.96, and 0.97 and 0.87, respectively. DEXOH in the pooled HLM (32.6 pmol/mg protein/min) was approximately 5-fold higher than that in uPA+/-/SCID mice (6.2 pmol/mg of protein/min) and uPA-/-/SCID mice (7.2 pmol/mg protein/min). This activity in microsomes from donor A (12.5 pmol/mg protein/min) was 38% compared with that in the pooled HLM.
Human CYP3A4 expression in the chimeric mice. Relative expression levels of human CYP3A4 mRNA (A), the expression of human CYP3A4 protein (B), and DEXOH (C) were determined as described under Materials and Methods. C, DEXOH catalyzed by CYP3A4 measured at 100 μM dexamethasone. Each column represents the mean of duplicate determinations except in uPA+/-/SCID mice and uPA-/-/SCID mice. The columns of uPA+/-/SCID mice and uPA-/-/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
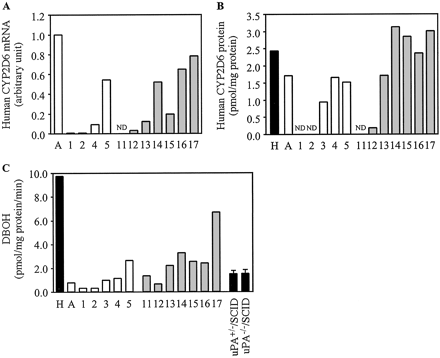
Expression of Human CYP2D6 in Chimeric Mice. The expression of human CYP2D6 mRNA, the expression of human CYP2D6 protein, and DBOH were increased in a hAlb concentration-dependent manner (Fig. 3). DBOH in the pooled HLM (9.7 pmol/mg protein/min) was approximately 6.5-fold higher than that in uPA+/-/SCID mice (1.5 pmol/mg protein/min) and uPA-/-/SCID mice (1.5 pmol/mg protein/min). This activity in microsomes from donor A was lower compared with that in the pooled HLM.
Human CYP2D6 expression in the chimeric mice. Relative expression levels of human CYP2D6 mRNA (A), the expression of human CYP2D6 protein (B), and DBOH (C) were determined as described under Materials and Methods. C, DBOH catalyzed by CYP2D6 measured at 5 μM debrisoquine. Each column represents the mean of duplicate determinations except in uPA+/-/SCID mice and uPA-/-/SCID mice. The columns of uPA+/-/SCID mice and uPA-/-/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
Expression of Human CYP2C8 in Chimeric Mice. The expression of human CYP2C8 mRNA, the expression of human CYP2C8 protein, and PTXOH were increased in a hAlb concentration-dependent manner (Fig. 4). PTXOH in uPA+/-/SCID mice and uPA-/-/SCID mice could not be detected in this experimental condition. This activity in microsomes from donor A (100.6 pmol/mg protein/min) was 45% compared with that in the pooled HLM (224.4 pmol/mg protein/min).
Human CYP2C8 expression in the chimeric mice. Relative expression levels of human CYP2C8 mRNA (A), the expression of human CYP2C8 protein (B), and PTXOH (C) were determined as described under Materials and Methods. C, PTXOH catalyzed by CYP2C8 measured at 20 μM paclitaxel. Each column represents the mean of duplicate determinations except in uPA+/-/SCID mice and uPA-/-/SCID mice. The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
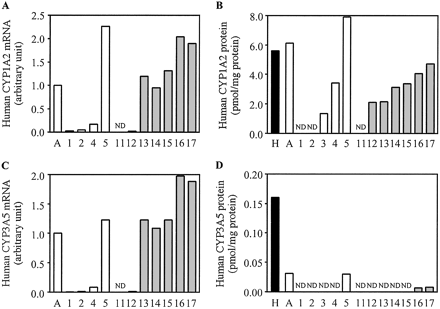
Expressions of Human CYP1A2 and CYP3A5 in Chimeric Mice. The expressions of human CY1A2 and CYP3A5 mRNA and the expressions of those proteins were increased in a hAlb concentration-dependent manner (Fig. 5). The content of human CYP1A2 and CYP3A5 protein in donor A was 1.1- and 0.2-fold higher than that in the pooled HLM, respectively. In donor A, the donor A chimeric mice, and the donor B chimeric mice, the content of human CYP3A5 protein was much lower than that in the pooled HLM.
Human CYP1A2 and CYP3A5 expressions in the chimeric mice. Relative expression levels of human CYP1A2 (A) and CYP3A5 (C) mRNA and human CYP1A2 (B) and CYP3A5 (D) protein were determined as described by Western blotting under Materials and Methods. The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
Expression of Human CYP2C19 in Chimeric Mice. The expression of human CYP2C19 mRNA and MPOH in the chimeric mice are shown in Fig. 6. The expression of human CYP2C19 mRNA in the chimeric mice was increased in a hAlb concentration-dependent manner. MPOH in the pooled HLM (47.0 pmol/mg protein/min) was approximately 8.4- and 2.7-fold higher than that in uPA+/-/SCID mice (5.6 pmol/mg protein/min) and uPA-/-/SCID mice (17.3 pmol/mg protein/min), respectively. MPOH activity in donor A (10.9 pmol/mg protein/min) was 23% compared with that in the pooled HLM. Only chimeric mouse 5 exhibited MPOH among the chimeric mice with hepatocytes from donor A, although this activity could be detected in the chimeric mice with hepatocytes from donor B. The content of human CYP2C19 protein could not be quantified because there were no applicable primary antibodies commercially available.
Human CYP2C19 expression in the chimeric mice. Relative expression levels of human CYP2C19 mRNA (A) and MPOH (B) were determined as described under Materials and Methods. B, MPOH catalyzed by CYP2C19 measured at 200 μM S-mephenytoin. Each column represents the mean of duplicate determinations except in uPA+/-/SCID mice and uPA-/-/SCID mice. The columns of uPA+/-/SCID mice and uPA-/-/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
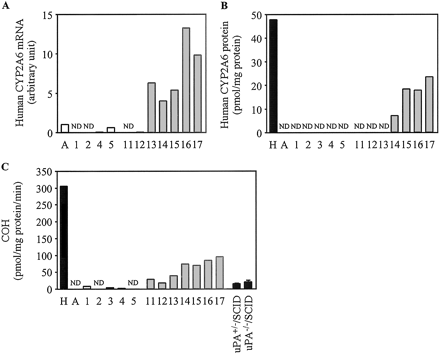
Expression of Human CYP2A6 in Chimeric Mice. The expression of human CYP2A6 mRNA, the expression of human CYP2A6 protein, and COH in the chimeric mice are shown in Fig. 7. In the case of the donor A chimeric mice, neither the human CYP2A6 protein nor COH could be detected. However, in the donor B chimeric mice, the expression of human CYP2A6 protein and COH were increased in a hAlb concentration-dependent manner. COH in the pooled HLM (305.4 pmol/mg protein/min) was approximately 20.2- and 15.1-fold higher than in uPA+/-/SCID mice (15.1 pmol/mg protein/min) and uPA-/-/SCID mice (20.2 pmol/mg protein/min), respectively.
Human CYP2A6 expression in the chimeric mice. The relative expression levels of human CYP2A6 mRNA (A), the expression of human CYP2A6 protein (B), and COH (C) were determined as described under Materials and Methods. C, COH catalyzed by CYP2A6 measured at 1 μM coumarin. Each column represents the mean of duplicate determinations except in uPA+/-/SCID mice and uPA-/-/SCID mice. The columns of uPA+/-/SCID mice and uPA-/-/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 2. H, HLM; A, donor A; ND, not detected.
Genotyping of Human CYP2A6, Human CYP2C19, and Human CYP3A5 Alleles. By using genomic DNA extracted from liver, donor A and the chimeric mice with hepatocytes from the same donor were genotyped for CYP2A6*4, CYP2C19*2, and CYP2C19*3. Both donor A and the chimeric mice were genotyped as CYP2A6*4/CYP2A6*4 and CYP2C19*1/CYP2C19*2 (data not shown). Similarly, donor A, the donor A chimeric mice, and the donor B chimeric mice were genotyped for CYP3A5*3. All were genotyped as CYP3A5*3/CYP3A5*3 (data not shown).
Discussion
P450 is a superfamily of the heme-containing protein that is involved in the metabolism of endogenous substrates and xenobiotics including therapeutic drugs (Li et al., 1995). Some groups have successfully established transgenic mice with human P450s such as CYP2D6 and CYP3A4 (Corchero et al., 2001; Robertson et al., 2003; Zhang et al., 2003), which could be used to assess the toxicity of chemicals and to study the transcriptional regulation of the P450 genes. Since transgenic mouse models usually express only one human P450 with many murine P450s still retaining normal activities, their usefulness for predicting drug metabolism in humans in vivo is limited. On the other hand, human hepatocytes and hepatoma cell lines are frequently used as in vitro systems to investigate drug metabolism. Human hepatocytes retain near-normal hepatocellular morphology and the expression of the entire hepatic drug-metabolizing enzyme system in an integrated form. However, the drug-metabolizing capacity in human hepatocytes decreases during culture due to a decrease of the P450 gene transcription (Rodrìguez-Antona et al., 2002). Hepatoma cell lines have unlimited life span but very low P450 expression (Guillouzo, 1998). Recently, chimeric mice whose livers could be replaced by more than 80% with human hepatocytes were established by Tateno et al. (2004). In the present study, we investigated the major human hepatic P450s in detail in these chimeric mice in terms of the mRNA, protein, and enzyme activity.
The expression of human CYP2C9 mRNA, the expression of CYP2C9 protein, and DICOH tended to correlate with the hAlb concentrations. The expression of human P450 in the chimeric mice could be monitored by the specific enzyme activity, catalyzed by human P450 but not by murine P450. DICOH was reported to be detectable in humans but not in mice (Mankowski et al., 2000). The Vmax values of DICOH in humans were reported to be 10- or 20-fold higher than those in mice, although the Km values were similar (Bogaards et al., 2000). In this study, DICOH in humans was approximately 20-fold higher than that in mice, consistent with previous reports. The investigation of DICOH in liver microsomes is suggested to be a more suitable way to distinguish human P450 from murine P450. DICOH in chimeric mouse 5 was approximately 2-fold higher than that in donor A, although the contents of CYP2C9 protein were similar. The enzyme activity in donor A microsomes might become lower during the preparation and/or storage, or human CYP2C9 protein might have been easy to express in chimeric mouse 5. The reason remains to be clarified.
The expression of human CYP3A4, human CYP2D6, and human CYP2C8 demonstrated a similar tendency to that of CYP2C9 in terms of the mRNA, protein, and DEXOH, DBOH, or PTXOH. Dexamethasone is primarily metabolized to 6-hydroxydexamethasone in humans but to 6-hydroxy-9α-fluoro-androsta-14-diene-11β-hydroxy-16α-methyl-3,17-dione in mice (Tomlinson et al., 1997). DBOH is a typical reaction catalyzed by human CYP2D6, but lower DBOH was exhibited in the various strains of mice (Masubuchi et al., 1997). PTXOH has been reported the species difference in in vitro study using human, rat, minipig, and pig microsomes (Vaclavikova et al., 2004). DEXOH, DBOH, and PTXOH in the microsomes of uPA+/-/SCID mice or uPA-/-/SCID mice were lower compared with the pooled HLM. DEXOH in donor A was lower than that in the pooled HLM; thus, these activities in all of the donor A chimeric mice were low, thereby diminishing the differences between humans and mice. In CYP2D6 and CYP2C8, the enzyme activity in chimeric mouse 5 was higher than that in donor A, although the contents of protein were similar, as in CYP2C9.
From the results of CYP2C9, CYP3A4, CYP2D6, and CYP2C8, if mRNA and protein were expressed, human P450 would exhibit the drug metabolism potency in the chimeric mouse liver. Although we could not determine the specific activities catalyzed by human CYP1A2 and CYP3A5, mRNA and the protein of human CYP1A2 and CYP3A5 were detectable in a hAlb concentration-dependent manner. Among all human P450s, the mean r values between mRNA, protein, or enzyme activity and a hAlb concentration were 0.82, 0.93, and 0.87, respectively. Therefore, these human P450s also retained the catalytic activity of drug metabolism. It is difficult to determine whether the ratio of each P450 content in the liver of a chimeric mouse would be the same as that of the donor. Further studies using various donors are needed to clarify this point. From our data, the amount of mRNA, the protein content, and the enzyme activity would be similar between the donor and chimeric mice with hepatocytes from the same donor with a higher concentration of hAlb.
The uPA+/+/SCID mice showed hepatic failure due to proteolytic damage and could not remain alive long. In chimeric mice no. 1 and no. 12 (12-14 weeks old), some P450 activities were detected, which might be due to the partial repopulation of murine hepatocytes rather than human hepatocytes.
Another important aspect in drug metabolism for which the chimeric mice are useful is in reflecting the human polymorphic phenotypes and genotypes. Polymorphisms of P450s can cause adverse effects and interindividual variability in the metabolism of drugs in humans. It is important to confirm whether the chimeric mice have the same genotype as the donor. Until now, several CYP2A6 alleles have been reported (Nakajima et al., 2002a). One of the alleles, CYP2A6*4, deletes the whole CYP2A6 gene. Both donor A and the donor A chimeric mice were genotyped as CYP2A6*4/CYP2A6*4 using genomic DNA extracted from liver. It is reasonable that the donor A chimeric mice could not detect CYP2A6 protein and COH, whereas the donor B chimeric mice exhibited CYP2A6 protein and COH. It has been reported that the Vmax value of COH in humans was 25-fold higher than that in mice (Bogaards et al., 2000), which is consistent with the result of the present study (15-20-fold). COH is also suitable for estimating the humanization of the chimeric mice as DICOH.
The phenotype of CYP2C19 as a poor metabolizer correlates with the CYP2C19 genotype when any combination of CYP2C19 alleles CYP2C19*2, CYP2C19*3, CYP2C19*4, CYP2C19*5, CYP2C19*6, CYP2C19*7, and CYP2C19*8 are present (Wedlund, 2000). Two major mutations, CYP2C19*2 and CYP2C19*3, account for almost 100% of poor metabolizers in the Japanese population (de Morais et al., 1994). Both donor A and the donor A chimeric mice were genotyped as CYP2C19*1/CYP2C19*2 using the genomic DNA extracted from liver. Therefore, the lower MPOH activities in the donor A chimeric mice might be due to the genetic polymorphism.
In the chimeric mice with hepatocytes from donor A and donor B, the expression of human CYP3A5 protein was very low, suggesting that the expression of CYP3A5 protein would be lower in both donors. The genotype of donor A, the donor A chimeric mice, and the donor B chimeric mice were homozygous for the CYP3A5*3 allele, which was clarified to decrease CYP3A5 protein compared with CYP3A5*1 (Kuehl et al., 2001). The lower CYP3A5 protein levels were due to the genetic polymorphism. Taking these points into consideration, it is noteworthy to confirm that the chimeric mice retained the genotype and phenotype of the donor.
In conclusion, the livers of the chimeric mice used in the present study expressed human P450s and exhibited a similar capacity for drug metabolism in humans. Moreover, the liver in the chimeric mice exhibited the same genotype and phenotype as the donor, indicating that the interindividual variability due to the genetic polymorphism could be evaluated using the data from the chimeric mice. The metabolic activity in the chimeric mice with higher hAlb concentrations may be catalyzed by human P450s, and there is minimal influence by murine P450s. Further study is needed to clarify the induction and inhibition of human P450s and the expressions of phase II enzymes and transporters. In our laboratory, these studies are underway. The chimeric mice with humanized liver would become a useful model in studies of drug metabolism. We hope that the present study will contribute to the future study in drug development.
Acknowledgments
We acknowledge Brent Bell for reviewing the manuscript.
Footnotes
-
This work was supported by a Research on Advanced Medical Technology, Health, and Labor Sciences Research grant from the Ministry of Health, Labor, and Welfare of Japan.
-
doi:10.1124/dmd.104.001347.
-
ABBREVIATIONS: P450, cytochrome P450; HLM, human liver microsomes; uPA, urokinase-type plasminogen activator; SCID, severe combined immunodeficient; hAlb, human albumin; RI, replacement index; PCR, polymerase chain reaction; COH, coumarin 7-hydroxylase activity; HPLC, high-performance liquid chromatography; PTXOH, paclitaxel 6α-hydroxylase activity; DICOH, diclofenac 4′-hydroxylase activity; MPOH, S-mephenytoin 4′-hydroxylase activity; DBOH, debrisoquine 4′-hydroxylase activity; DEXOH, dexamethasone 6-hydroxylase activity.
- Received July 4, 2004.
- Accepted September 14, 2004.
- The American Society for Pharmacology and Experimental Therapeutics