Abstract
A systematic kinetic analysis of the metabolism of five benzodiazepines (low to high clearance compounds) was performed in CYP3A4, CYP3A5, and CYP2C19 baculovirus-expressed recombinant systems. The data obtained in the expression systems were scaled and compared with human liver microsomal predicted clearance and observed in vivo values, using either cytochrome P450 relative activity factors (RAFs) or the relative abundance approach. Interindividual variability, both in content (CYP3A4, CYP3A5) and activity (CYP3A4, CYP3A5, and CYP2C19), were incorporated in the clearance prediction by bootstrap analysis. These resampling Monte Carlo-based simulations were performed to justify any distribution assumptions in the generated range of the predicted clearance due to a limited sample size. This approach allowed extrapolation of the recombinant clearance data to specific population groups and investigation of the role of “minor” forms like CYP3A5 and CYP2C19 in comparison to the most prolific CYP3A4. The use of quinidine 3-hydroxylation and alprazolam 1′-hydroxylation as RAF markers for CYP3A4 and CYP3A5 activity, respectively, and the incorporation of variability improved the clearance prediction of the selected benzodiazepines (apart from flunitrazepam) to within 2-fold of the in vivo value. Clearance estimates from the immunoquantified protein levels were approximately 8-fold lower in comparison to the RAF approach. The differences observed in the benzodiazepine metabolite pathway ratios between CYP3A4 and CYP3A5, particularly for 1′- to 4-hydroxymidazolam and alprazolam, provided a useful measure of interindividual differences within the CYP3A family.
Recombinant cytochrome P450 enzymes (rP450s) are a convenient and successful in vitro tool for the identification of the metabolic pathway(s) and the quantification of the individual P450 enzyme(s) contributing to the overall metabolism of a drug (Crespi, 1995; Ito et al., 1998, Nakajima et al., 2002). In a large number of cases, CYP3A enzymes are found to be the enzyme of paramount importance. However, recent studies have placed emphasis on other CYP3A family members, notably CYP3A5, which demonstrate either similar or reduced metabolic activity in comparison to CYP3A4 (Williams et al., 2002; Patki et al., 2003), and the clinical significance of hepatic and intestinal CYP3A5 is currently under debate (Paine et al., 1997; Lin et al., 2002; Thummel, 2003; Westlind-Johnsson et al., 2003; Wong et al., 2004; Xie et al., 2004).
Recombinant enzymes also represent an alternative in vitro metabolic system to hepatic microsomes or hepatocytes to predict the in vivo clearance of drugs (Ito et al., 1998; Hirota et al., 2001). This approach offers an advantage in allowing incorporation and customization of interindividual variation of P450 expression, frequently a confounding element in human prediction from hepatic microsomes, particularly for CYP3A substrates. In many microsomal prediction studies, interindividual variability issues are ignored because predictions are based on kinetic data generated in a limited number of livers or using a “representative” liver pool rather than a characterized liver bank. Approaches to introduce population variability in P450 expression into in vivo predictions from hepatic tissues have yet to be established.
Recently, a number of authors have questioned the degree of CYP3A variability in vivo. A collated database of 17 independent studies by Lin et al. (2001) indicated only a 4-fold interindividual variability in 85% of healthy subjects. Floyd et al. (2003) observed a similar degree of variability with the probe midazolam, regardless of the route of administration, reflecting, therefore, hepatic (intravenous route) and combined hepatic and intestinal activity (oral route). However, one could argue that bias in the estimate of variability due to strict study criteria (healthy volunteers, low dose of midazolam, certain ethnic groups) could reduce the incidence of extreme data. In addition to intrinsic variability, in vitro differences between the liver banks can be associated with storage and degradation during harvesting (possible differential degradation between CYP3A4 and CYP3A5 indicated by Floyd et al., 2003). Therefore, it is not surprising that the range of midazolam CLint estimates have been reported to differ 5-fold between two liver banks (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication). In addition, the contribution of both enzymes is rarely included in the actual prediction of clearance of CYP3A substrates (Hirota et al., 2001). Both relative activity factors (RAFs) and relative abundance of certain P450s have been proposed for the quantitative prediction of clearance from heterologous expression systems (Störmer et al., 2000; Venkatakrishnan et al., 2000). The application of both approaches in clearance prediction and the selection of the appropriate marker substrate for determination of RAF, both for CYP3A4 and CYP3A5, are assessed here.
The overall aim of the current study is to investigate the utility of recombinant enzymes for human clearance prediction and to incorporate a range of complex issues associated with CYP3A: well documented atypical kinetics (Galetin et al., 2003), and the importance of parallel pathways and multiple P450 involvement, in particular the contribution of two polymorphic, “minor” enzymes (CYP3A5 and CYP2C19). Additionally, approaches to introduce interindividual variability in clearance estimates obtained by both RAF and P450 abundance approaches are investigated and compared with the prediction obtained using human liver microsomes and in vivo data collated from a number of clinical studies (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication). Five benzodiazepines, midazolam, diazepam, triazolam, flunitrazepam, and alprazolam, were selected due to the 100-fold difference in their microsomal clearance, the availability of an extensive in vivo database previously collated in our laboratory (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication) and the involvement of CYP3A4, CYP3A5 and CYP2C19 in their metabolism (Bertilsson et al., 1989, Andersson et al., 1994, Gorski et al., 1994, Hesse et al., 2001, Hirota et al., 2001).
Materials and Methods
Chemicals. Midazolam, triazolam, alprazolam, diazepam, flunitrazepam, quinidine, mephenytoin, NADP, isocitric dehydrogenase were purchased from Sigma Chemicals Co. (Poole, Dorset, UK). (3S)-3-Hydroxyquinidine, 4′-hydroxymephenytoin and midazolam metabolites were obtained from Ultrafine Chemicals (Manchester, UK). Alprazolam and triazolam metabolites were purchased from Biomol International (PA, USA) and flunitrazepam metabolites were a gift from Roche (Basel, Switzerland). All other reagents and solvents were of high analytical grade. Microsomes from baculovirus-insect-cell-expressed CYP3A4 with coexpressed NADPH-cytochrome P-450 reductase (CYP3A4/oxidoreductase) were obtained from BD Gentest Co. (Woburn, MA, USA).
Incubation Conditions. The kinetic studies were performed in Supersomes, baculovirus-insect cell-expressed systems, containing no cytochrome b5. Incubation times (5 min for midazolam, 10 min for mephenytoin, and 20 min for all the other benzodiazepines and quinidine) and protein concentrations (0.05-0.2 mg/ml) were within the linear range for each individual substrate. Protein concentration was equivalent to the relative content of 13.5 to 22 pmol of P450/incubation system, and the equivalent amounts of all three enzymes were used. Microsomes were suspended in phosphate buffer (0.1 M, pH 7.4) with a final incubation volume of 0.25 ml. Samples were preincubated for 5 min in a shaking water bath at 37°C, and each reaction was initiated with an NADPH regenerating system (1 mM NADP+, 7.5 mM isocitric acid, 10 mM magnesium chloride, and 0.2 unit of isocitric dehydrogenase). No significant microsomal binding was observed (<10%) for either of the substrates investigated. The final concentration of the organic (either methanol or acetonitrile) in incubation media was 0.1% v/v. The substrate concentrations ranged from 2.5 to 1000 μM (alprazolam, triazolam), 2.5 to 500 μM (diazepam), 10 to 1000 μM (flunitrazepam, mephenytoin), 1 to 200 μM (midazolam), and 1 to 500 μM (quinidine). The reaction was terminated by 0.25 ml of ice-cold acetonitrile with a 1 μM concentration of the appropriate internal standard; samples were centrifuged at 13,400g for 10 min and further analyzed by liquid chromatography/tandem mass spectrometry.
Liquid Chromatography/Tandem Mass Spectrometry Methods. Each metabolite pair, together with either diazepam (for 1′- and 4-hydroxyalprazolam and triazolam), triazolam (for 3-hydroxy and nordiazepam), clobazam (for 3-hydroxy and desmethylflunitrazepam), alprazolam (for 1′- and 4-hydroxymidazolam), or dextromethorphan (for 3-hydroxyquinidine), as internal standard, were separated on a Luna C18(2) 50 × 4.6 mm, 3-μm column (Phenomenex, Macclesfield, UK) at 40°C using either a binary or ternary gradient maintained at 1 ml/min by a Waters Alliance 2795 HT LC system.
For 1′- and 4-hydroxyalprazolam, an initial mobile phase of 90% 0.001 M ammonium acetate/10% acetonitrile was ramped immediately to 66% 0.001 M ammonium acetate/34% acetonitrile at 1 min and immediately to 34% 0.001 M ammonium acetate/66% acetonitrile at 4 min. The initial ratio was immediately re-established at 5 min and maintained to 6 min. The retention times were approximately 4.1 (4-hydroxyalprazolam), 4.4 (1′-hydroxyalprazolam), and 5.7 (diazepam) min. For 3-hydroxy and nordiazepam, an initial mobile phase of 90% 0.001 M ammonium acetate/10% acetonitrile was ramped linearly to 18% 0.001 M ammonium acetate/82% acetonitrile from 1 to 4 min. The initial ratio was immediately reestablished at 4 min and maintained to 5 min. The retention times were approximately 4.2 (triazolam) and 4.4 (3-hydroxy, nordiazepam) min.
For 3-hydroxy and desmethylflunitrazepam, an initial mobile phase of 90% 0.001 M ammonium acetate/10% acetonitrile was ramped linearly to 10% 0.001 M ammonium acetate/90% acetonitrile from 1 to 5 min. The initial ratio was immediately reestablished at 5 min and maintained to 5.5 min. The retention times were approximately 4.3 (3-hydroxy, desmethylflunitrazepam) and 4.7 (clobazam) min. For 1′- and 4-hydroxymidazolam, an initial mobile phase of 66% 0.001 M ammonium acetate/34% acetonitrile was ramped linearly to 50% 0.001 M ammonium acetate/50% acetonitrile between 1 and 4 min. The initial ratio was immediately reestablished at 4 min and maintained to 5 min. The retention times were approximately 3.2 (4-hydroxymidazolam), 3.3 (alprazolam), and 3.5 (1′-hydroxymidazolam) min. For 1′- and 4-hydroxytriazolam, an initial mobile phase of 90% 0.001 M ammonium acetate/10% acetonitrile was ramped immediately to 66% 0.001 M ammonium acetate/34% acetonitrile at 1 min and immediately to 34% 0.001 M ammonium acetate/66% acetonitrile at 4 min. The initial ratio was immediately reestablished at 5 min and maintained to 6.5 min. The retention times were approximately 4.4 (1′-hydroxytriazolam), 4.5 (4-hydroxytriazolam), and 5.7 (diazepam) min. For 3-hydroxyquinidine, an initial mobile phase of 90% 0.001 M ammonium acetate/10% acetonitrile was ramped linearly to 90% 0.01 M formic acid/10% acetonitrile between 1 and 4 min. The initial ratio was immediately reestablished at 5 min and maintained to 6 min. The retention times were approximately 2.9 (3-hydroxyquinidine) and 3.2 (dextromethorphan) minutes.
The compounds were detected and quantified by atmospheric pressure electrospray ionization MS/MS using a Waters Micromass Quattro Ultima triple quadrupole mass spectrometer (Waters Micromass MS Technologies Ltd., Manchester, UK). The LC column eluate was split and one-fourth was delivered into the mass spectrometer where the desolvation gas (nitrogen) flow rate was 600 l/h, the cone gas (nitrogen) flow rate was 100 l/h, and the source temperature was 125°C. Using positive ion mode, protonated molecular ions were formed using a capillary energy of 3.5 kV and cone energies of 39 V (3-hydroxydiazepam), 70 V (diazepam, 3-hydroxyquinidine), 71 V (3-hydroxyflunitrazepam), 74 V (nordiazepam), 76 V (desmethylflunitrazepam), 78 V (clobazam), 80 V (1′- and 4-hydroxymidazolam, 1′- and 4-hydroxytriazolam, triazolam), 89 V (dextromethorphan), and 90 V (alprazolam). Product ions formed in argon at a pressure of 2 × 10-3 mbar and at collision energies of 12 eV (3-hydroxydiazepam, m/z 301.1→254.9), 15 eV (3-hydroxyflunitrazepam, m/z 330.05→284.35), 20 eV (4-hydroxyalprazolam, m/z 325.05→280.10; diazepam, m/z 285.0→257.00; clobazam, m/z 301.05→259.35), 22 eV (desmethylflunitrazepam, m/z 300.00→254.35), 25 eV (nordiazepam, m/z 270.95→208.00; 4-hydroxymidazolam, m/z 342.00→234.30; alprazolam, 309.00→281.30; triazolam, 343.00→308.00), 28 eV (3-hydroxyquinidine, 341.05→226.05), 30 eV (1′-hydroxyalprazolam, m/z 325.05→297.1; 1′-hydroxymidazolam, m/z 342.00→203.30; 1′-hydroxytriazolam, m/z 359.05→176.0), 35 eV (4-hydroxytriazolam, m/z 359.05→273.00), and 40 eV (dextromethorphan, 272.05→170.90) were monitored as ion chromatograms which were integrated and quantified by quadratic regression of standard curves using Micromass QuanLynx 3.5 software.
Quantitative Western Blotting. The absolute amounts of CYP3A4 and CYP3A5 in 12 tissue samples from HL Bank 2 were measured by SDS-polyacrylamide gel electrophoresis in 12% precast gel (Bio-Rad, Hercules, CA), followed by Western blotting with specific anti-CYP3A4 and -CYP3A5 antibodies (BD Gentest, Woburn, MA). Heterologously expressed CYP3A4 and CYP3A5 in baculovirus (BD Gentest) were used for calibration. CYP3A protein levels were estimated by comparison of the sample band integrated optical density with the appropriate standard curve, with a limit of quantitation of 10 and 1 pmol/mg protein for CYP3A4 and CYP3A5, respectively.
Data Analysis. The kinetic parameters for each substrate were obtained from untransformed data by nonlinear least-squares regression using GraFit 5 (Erithacus Software, Horley, Surrey, UK). In the case of 3-hydroxyquinidine, 1′- and 4-hydroxymidazolam (CYP3A4), flunitrazepam N-demethylation (CYP3A5), and 3-hydroxylation (CYP2C19), the Michaelis-Menten equation with a weighting factor of 1/y was used for kinetic analysis. Kinetic parameters Vmax, Ks (substrate dissociation constant), α (defining changes in binding affinity - homotropic cooperativity), and β (changes in catalytic rate constant, Kp) were calculated from untransformed data using the two-site model (Houston et al., 2003). This type of analysis was performed for the cases of positive and negative cooperativity, i.e., sigmoidal and substrate inhibition kinetic profiles, respectively. When the metabolic profile was consistent with positive homotropic behavior, the CLmax, the maximum clearance when the enzyme is fully activated, was calculated by the following equation: 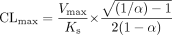
The CLmax estimate was derived from the two-site kinetic model (see Appendix), assuming that β = 2 [Vmax is equivalent to 2Kp[E]t, where [E]t is the total enzyme concentration (Segel, 1975)].
Clearance Prediction from rP450: Relative Abundance Approach. Immunoquantified levels of CYP3A4 and CYP3A5 enzymes in an HL Bank of 12 livers (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication) were used to predict the clearance by the following equation: 
No information on the CYP2C19 abundance in this HL Bank was available and, therefore, this enzyme was not included in the prediction via this approach.
Relative Activity of P450s. To obtain meaningful quantitative comparison with the data for human liver microsomes (HLM), Crespi (1995) has suggested the utilization of RAF, which represents the ratio of the rate of a specific marker reaction in HLM to the rate of the same metabolic pathway catalyzed by the specific cDNA-expressed isoform (eq. 3). Although this activity-based approach enables the correlation between the two systems, it assumes the possibility of extrapolation from the marker substrate to the other substrates for the same isoform, which can be questionable for CYP3A4. In contrast to the P450 content, the RAF approach accounts for differences in activity per unit enzyme between recombinants and HLM and may therefore represent a better scaling estimate than immunoquantified protein levels. Similar substrate specificity between CYP3A enzymes and a not yet defined specific marker for CYP3A5 represents an additional complicating issue. 
The RAF value obtained can be expressed either in units of nmol of P450/mg protein or, if the abundance of a particular P450/mg protein is incorporated, as unitless values, with the overall calculation of CLpred being the same.
Clearance Prediction from rP450: Relative Activity Approach. Based on the metabolite formation observed in both rCYP3A4 and CYP3A5, quinidine 3-hydroxylation was used as a selective CYP3A4 marker and the RAF was calculated using either our human liver bank or the BD Gentest liver pool as liver sources. Alprazolam 1′-hydroxylation was the most sensitive probe to generate CYP3A5 RAF, as described under Discussion. The CYP2C19 RAF estimate using the Vmax approach (same as for all the other isoforms) was determined using mephenytoin 4′-hydroxylation.
Contribution of a particular enzyme to the overall CL is calculated as shown in eq. 4 (Ito et al., 1998); the units for CLrP450 and RAFP450 are μl/min/nmol P450 and nmol of P450/mg protein, respectively. 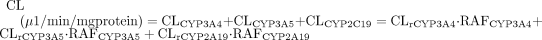
In addition to CYP3A4, the contribution of CYP3A5 and CYP2C19 (where applicable) to the formation of both 1′- and 4-hydroxy (midazolam, alprazolam, and triazolam) and 3-hydroxy and N-demethyl (diazepam and flunitrazepam) was incorporated by bootstrapping (Armitage et al., 2002). This resampling analysis with replacement (1000 simulations in S-Plus 2000; Mathsoft Inc., Cambridge, MA) was performed to obtain an estimate of the standard error of the predicted clearance due to relatively small initial sample size (n = 12).
Bootstrap-predicted clearance values from both the RAF and abundance approaches (mean ± S.D.) were scaled to in vivo clearance, applying a physiologically based scaling factor of 856 mg of protein/kg, obtained as the average recovery of microsomal protein per gram of liver (40 mg of protein/g liver), determined using 38 human livers, multiplied by the average liver weight in humans (21.4 g of liver/kg) (K. Ito and J. B. Houston, manuscript submitted for publication). A literature database of in vivo CLint values for benzodiazepines used to assess predictions and variability was collated from plasma clearances reported for 4 to 20 data sets and 38 to 237 individual subjects per drug (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication).
Results
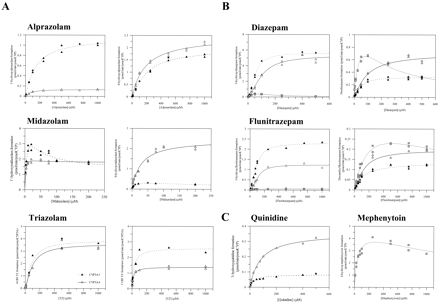
General Kinetics of Benzodiazepines in rP450s. The kinetic properties of five benzodiazepines were determined in human CYP3A4, CYP3A5, and CYP2C19 Supersomes with coexpressed oxidoreductase (Tables 1 and 2). Less than 10% substrate depletion was noted, and secondary metabolism was minimal throughout the course of the incubation. In each case, two metabolites were formed but with substantial quantitative differences between P450s.
Kinetic parameters for midazolam, triazolam and alprazolam metabolite formation in CYP3A4 and CYP3A5 baculosomes (mean ± S.E.)
Kinetic parameters for diazepam and flunitrazepam metabolite formation in CYP3A4, CYP3A5, and CYP2C19 baculosomes (mean ± S.E.)
Positive cooperativity, commonly reported with CYP3A4 (Houston et al., 2003), was also observed in the kinetic profiles obtained with CYP3A5 for alprazolam, 4-hydroxytriazolam, and 3-hydroxylation of diazepam and flunitrazepam. However, negative cooperativity (substrate inhibition) was observed for 1′- and 4-hydroxymidazolam (CYP3A5) and mephenytoin 4′-hydroxylation and diazepam and flunitrazepam N-demethylation with recombinant CYP2C19, but not CYP3A4. Kinetic profiles obtained for quinidine for both CYP3A enzymes showed standard Michaelis-Menten kinetics, whereas 4-hydroxyalprazolam and both pathways for midazolam were hyperbolic only in CYP3A4 (Fig. 1). Two-site kinetic analysis of the atypical profiles showed that the extent of cooperative binding to the CYP3A5 binding site was similar to CYP3A4 for most of the substrates investigated (comparable α values, Table 1 and 2). The only exception was diazepam, where a higher affinity for the second substrate molecule was observed for both 3-hydroxy- and N-demethylation pathways in comparison to CYP3A4, resulting in higher clearance via CYP3A5.
Comparison of CYP3A4 and CYP3A5 metabolite formation profiles for six substrates. A, CYP3A4 (▵) and CYP3A5 (▴) substrates: alprazolam, midazolam, triazolam (1′-hydroxy and 4-hydroxy pathways). B, CYP3A4 (▵), CYP3A5 (▴), and CYP2C19 ( ) substrates: diazepam and flunitrazepam (3-hydroxy and N-demethylation pathways). C, quinidine-RAF marker for CYP3A4, where ▵ and ▴ represent CYP3A4 and CYP3A5, respectively; and mephenytoin-RAF marker for CYP2C19 activity. Data points represent the mean of duplicate determinations.
) substrates: diazepam and flunitrazepam (3-hydroxy and N-demethylation pathways). C, quinidine-RAF marker for CYP3A4, where ▵ and ▴ represent CYP3A4 and CYP3A5, respectively; and mephenytoin-RAF marker for CYP2C19 activity. Data points represent the mean of duplicate determinations.
In contrast to the positive homotropy observed with CYP3A4 and CYP3A5, both diazepam pathways and flunitrazepam N-demethylation with CYP2C19 were consistent with substrate inhibition (Fig. 1B). Of the substrates investigated, diazepam pathways showed the most pronounced substrate inhibition with the increasing substrate concentrations by CYP2C19 (β < 0.1), in contrast to a 0.22 to 0.45 range obtained for flunitrazepam N-demethylation, mephenytoin 4′-hydroxylation, and midazolam 1′- and 4-hydroxylation.
Midazolam, triazolam, and 4-hydroxyalprazolam metabolite formation by CYP2C19 was insignificant in comparison to both CYP3A enzymes (maximum of 0.2-4% of the rate obtained by CYP3A4/A5 over a range of substrate concentrations). In the case of 1′-hydroxyalprazolam formation, CYP2C19 activity at very low substrate concentrations (e.g., 10 μM) was comparable with CYP3A4, whereas the rates generated with CYP3A5 were over 2-fold higher. However, with the increasing alprazolam concentrations, the contribution of CYP2C19 was minor in comparison to CYP3A enzymes.
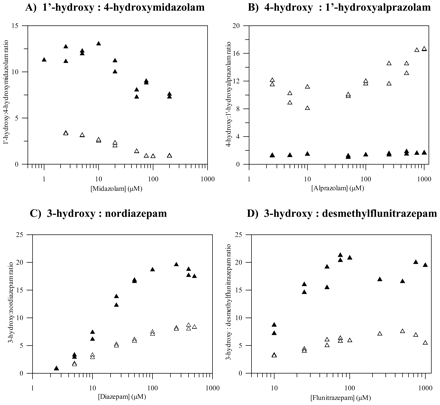
Pathway Ratio. Marked differences in pathway ratios (major/minor) between CYP3A5 and CYP3A4 (2- to 10-fold) were observed for all benzodiazepines except for triazolam; these differences showed noticeable substrate concentration dependence for diazepam and flunitrazepam (Fig. 2). The most characteristic changes in pathway ratios between CYP3A4 and CYP3A5 were observed for midazolam and alprazolam; over the range of substrate concentrations, the 1′- to 4-hydroxymidazolam ratio increased 4- to 10-fold. However, alteration in alprazolam regioselectivity was observed since the ratio of 4- to 1′-hydroxyalprazolam decreased in CYP3A5 (5- to 10-fold) in comparison with CYP3A4. The latter represented the only case of a pathway switch, i.e., the minor pathway by CYP3A4 was the dominant one by CYP3A5, since in other cases regioselectivity was maintained.
Comparison of metabolic pathway ratios for four benzodiazepines using CYP3A4 (▵) and CYP3A5 (▴). No significant difference between the P450s was observed for triazolam (data not shown).
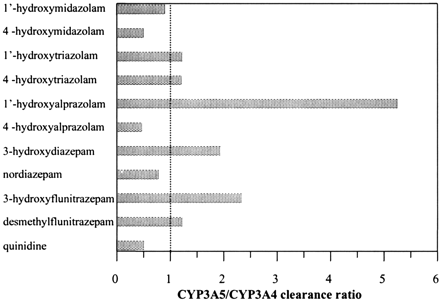
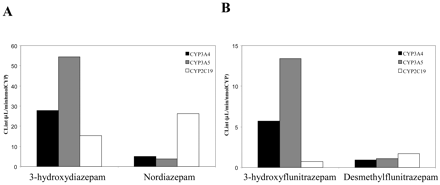
Clearance by Recombinant P450s. The ratio of clearances in recombinant CYP3A5 and CYP3A4, calculated using either CLint or CLmax (eq. 1), where appropriate, varied between substrates (Fig. 3). Clearance obtained for 3-hydroxyquinidine, 4-hydroxyalprazolam, 4-hydroxymidazolam, and nordiazepam formation was 1.3- to 2.3-fold higher in CYP3A4, whereas for the six other metabolic pathways, clearance in recombinant CYP3A5 exceeded clearances in recombinant CYP3A4 by 1.2- to 5.2-fold. Comparable activity between CYP3A4 and CYP3A5 was observed for 1′-hydroxymidazolam formation. Higher binding affinity for CYP3A5 (e.g., 3-hydroxydiazepam), higher Vmax values (1′- and 4-hydroxytriazolam), or a combination of both effects (3-hydroxyflunitrazepam) were responsible for the higher clearance observed by recombinant CYP3A5. 3-Hydroxylation of diazepam and flunitrazepam by CYP3A5 was dominant (55 and 68%, respectively), whereas CYP2C19 was more significant for N-demethylation (Fig. 4, A and B, for diazepam and flunitrazepam, respectively).
CYP3A5 to CYP3A4 clearance ratio obtained in Supersomes for the range of the substrates investigated and their respective pathways.
Contribution of CYP3A4, CYP3A5, and CYP2C19 to diazepam (A) and flunitrazepam (B) clearance in rP450s.
Of the substrates investigated, quinidine and alprazolam showed the largest differential in the contribution of either CYP3A4 or CYP3A5 to the overall kinetic profile. The relative ratio of quinidine 3-hydroxylation in CYP3A4 to CYP3A5 increased more than 4-fold between 5 and 500 μM quinidine concentrations. Differences in clearance values (Fig. 3) were not that pronounced due to 3-fold higher binding affinity for CYP3A5 (34 versus 102 μM). In contrast, rate of alprazolam 1′-hydroxylation, particularly at higher substrate concentrations, was 10-fold higher by CYP3A5 than CYP3A4 (Fig. 1A), increasing the clearance ratio (Fig. 3). Based on metabolite formation observed with both recombinant CYP3As, quinidine 3-hydroxylation was used as a selective CYP3A4 marker, and the RAF values obtained in both liver sources are shown in Table 3. The higher RAF obtained using BD Gentest HL Pool was in agreement with the previous report by Störmer et al. (2000). Large interindividual variability in the CYP3A4 activity in our liver bank was substantiated by a 10-fold difference in the individual RAF values, consistent with a study by Venkatakrishnan et al. (2000). However, the resulting mean value for CYP3A4 RAF was 4-fold higher than the estimate by Venkatakrishnan et al. (2000) using diazepam as a CYP3A4 marker. This discrepancy could be attributed to the arguable specificity of previously used markers for CYP3A4 (Störmer et al., 2000; Venkatakrishnan et al., 2000). Alprazolam 1′-hydroxylation was the most sensitive probe to generate CYP3A5 RAF, not reported previously in the literature. The CYP2C19 RAF estimate was determined using mephenytoin 4′-hydroxylation (Vmax in rCYP2C19 = 4.95 ± 0.49 pmol/min/pmol P450) and was within the previously reported range of 0.9 to 10.5 pmol of P450/mg protein (Ito et al., 1998).
RAF estimates (nmol of P450/mg protein) for CYP3A4, CYP3A5, and CYP2C19 using two different liver sources
Comparison of Recombinant Enzymes and HLM. The predicted total clearance from the recombinant enzyme data incorporated each enzyme and both pathways for midazolam, triazolam, diazepam, flunitrazepam, and alprazolam metabolism, by applying either relative abundance or the RAF approach (eqs. 2 and 4, respectively). For all the substrates investigated, 10 to 40% higher clearance values were obtained using BD Gentest HL Pool as a liver source for RAF determination (data not shown). Using the activity approach, clearance estimates for flunitrazepam and diazepam increased by 26 and 29%, respectively, when all three enzymes were included in the prediction in comparison to clearance when only CYP3A4 was considered. Clearance predicted using the relative abundance of CYP3A4 and CYP3A5 in our human liver bank (0.4-14% of total CYP3A) indicated only a minor contribution of hepatic CYP3A5 to the overall clearance of the prototypical CYP3A substrates (e.g., midazolam, quinidine). The only exception was alprazolam 1′-hydroxylation, where the CYP3A5 contribution varied between 3 and 47%. The rank order of the predicted clearance estimates was RAF > HLM > relative abundance (metabolite formation data for five benzodiazepines in liver microsomes and the prediction to in vivo CLint is shown in H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication). For the benzodiazepines investigated, RAF clearance estimates were 2- to 8-fold higher than HLM with the exception of flunitrazepam, for which the predictions from HLM and by RAF were similar (6.0 and 6.3 μl/min/mg protein, respectively).
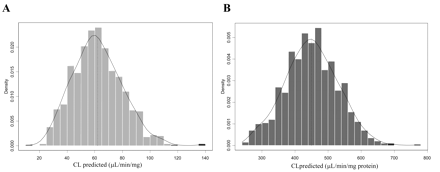
Clearance prediction from rP450 data for five benzodiazepines was performed by bootstrap analysis (1000 simulations). This resampling analysis was carried out to overcome the relatively limited information on CYP3A abundance and activity (data taken from n = 12), rationalize any distribution assumptions, and incorporate the variability of both P450 content and RAF estimates. An example of the bootstrap-generated distribution range of predicted clearance for midazolam and a 7.3-fold difference observed between the RAF and relative abundance approach is illustrated in Fig. 5
Predicted CL using relative abundance (A) and RAF (B) approach via bootstrap analysis: example of midazolam. Contributions of both 1′- and 4-hydroxy pathways via CYP3A4 and CYP3A5 are included.
Prediction of CLint in Vivo from Recombinant Systems. The bootstrap-generated range of predicted clearance with the incorporated variability of the relative abundance and P450 activity was scaled to clearance in vivo. Figure 6 illustrates the comparison of both scaled in vitro clearance (from rCYP3A4 and rCYP3A5 data) and in vivo CLint values (ml/min/kg) for five benzodiazepines investigated. Predicted clearance applying a range of immunoquantified CYP3A4 and CYP3A5 abundance (from human liver bank, n = 12) was 7- to 9-fold lower in comparison to the RAF approach. High variability in CYP3A4/CYP3A5 abundance in human liver bank was reflected in the high coefficients of variation of the CLpred obtained for each individual compound (28-99%); in contrast, variations by the RAF approach, although high, were more consistent (65-68%). For the benzodiazepines investigated, RAF clearance prediction was within 2-fold of the reported in vivo value with the exception of the 4-fold underestimation of flunitrazepam clearance.
Comparison of clearances predicted (mean ± S.D.) from recombinant enzymes and clearance observed in vivo for the five benzodiazepines. Interindividual variability in CYP3A4/CYP3A5 abundance and activity (RAF) was incorporated in the prediction by bootstrap analysis (1000 simulations). In vivo data used were from H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston (manuscript submitted for publication).
Discussion
To date, kinetic studies with recombinant enzymes have concentrated on achieving parity with hepatic microsomal preparations (Rodrigues, 1999; Störmer et al., 2000; Venkatakrishnan et al., 2000), with only a few reports of using such data for in vivo clearance predictions (Ito et al., 1998; Hirota et al., 2001; Proctor et al., 2004). We have expanded the predictive utility of recombinant enzyme kinetic parameters by incorporating frequency distribution of particular P450s and their activity as assessed by RAF estimates. An approach to building in variability to the scaling process (via either P450 abundance or activity approach) has been explored by using benzodiazepines as well characterized drugs extensively metabolized by CYP3A4. In addition, the potential impact of “minor” P450s (CYP3A5 and CYP2C19) and of the complex kinetic properties seen for all three enzymes has been assessed.
Kinetic Issues. The systematic kinetic analysis performed for five benzodiazepines showed no significant differences in substrate specificity between CYP3A4 and CYP3A5, in agreement with recent reports (Williams et al., 2002; Huang et al., 2003; Patki et al., 2003). However, for certain substrates, the relative CYP3A5 to CYP3A4 clearance ratio varied from previous reports; e.g., Williams et al. (2002) observed higher 4-hydroxytriazolam formation clearance by CYP3A4, whereas in our study, clearance by CYP3A5 was higher (Fig. 3). The use of a more precise method of clearance estimation [CLmax when the enzyme is fully activated (eq. 1), rather than the slope of the velocity curve at the low range of substrate concentrations applied by Williams et al. (2002)] may provide the possible explanation. Maximum clearance for 4-hydroxytriazolam was observed at a substrate concentration of 50 μM, and if consideration is not given to the phenomenon of activation, then clearance is likely to be underestimated. Clearance estimates obtained by both the two-site (CLmax) and the standard Michaelis-Menten approach (CLint) showed a wide range for CYP3A4 (0.9-728 μl/min/nmol P450) and CYP3A5 (1.1-626 μl/min/nmol P450).
The two-site mechanistic kinetic model used (Houston et al., 2003) showed that the atypical kinetic profiles were consistent between two enzymes for most of the pathways (Fig. 1, A and B). Positive cooperativity in substrate binding to the active site was apparent in CYP3A5 (Tables 1 and 2), supporting the existence of multiple binding sites on this enzyme comparable with CYP3A4.
Pathway Ratio. Regioselectivity of midazolam, diazepam, and flunitrazepam metabolic pathways was maintained in CYP3A5, but the relative pathway ratio was increased up to 10-fold in this enzyme relative to CYP3A4. A similar ratio has been reported for midazolam in both CYP3A5-positive livers (Gorski et al., 1994; Foti and Fisher, 2003) and intestines (Lin et al., 2002), indicating the value of this ratio as a measure of CYP3A4/CYP3A5 content in HLM. However, no significant correlation could be established between either the content or the relative contribution of CYP3A5 to the total CYP3A and the 1′- to 4-hydroxymidazolam ratio in our liver bank due to a small sample size. In addition, CYP3A5*3/*3 genotype was dominant in our livers, with only 4 of 12 livers heterozygous for the CYP3A5*1 allele and none with the most active CYP3A5*1/*1 genotype (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication). This is consistent with the findings by Lin et al. (2002) that the median “functional” CYP3A5 was 4-fold higher in CYP3A5*1/*3 livers than in CYP3A5*3/*3.
Our data on 3-hydroxy/desmethylflunitrazepam pathway ratios in CYP3A4 and CYP3A5 contradict the findings by Huang et al. (2003). However, the possible significance of the pathway ratio for flunitrazepam (and diazepam) is likely to be limited due to important involvement of CYP2C19 in N-demethylation.
Pathway regioselectivity was not maintained for all the benzodiazepines (Fig. 2). In the case of alprazolam, the switch in pathway importance was noted because the 1′-hydroxyalprazolam clearance by CYP3A5 was increased by 5.5-fold relative to CYP3A4, whereas a decrease by 2-fold was observed for the 4-hydroxylation. Hirota et al. (2001) have reported an increase in formation of 1′-hydroxyalprazolam, but to a smaller extent (3.4-fold). Due to the importance of CYP3A5 in 1′-hydroxyalprazolam formation observed in our study and in previous studies (Gorski et al., 1999; Hirota et al., 2001), this pathway was used as the most selective marker for CYP3A5 RAF estimation. A similar rationale was the basis for application of quinidine 3-hydroxylation to differentiate CYP3A4 from CYP3A5 activity.
Clearance Prediction from Recombinant Enzymes. The predicted clearance from immunoquantified P450 protein levels was up to 9-fold lower in comparison with the RAF approach for all the benzodiazepines studied; the extent of under-prediction was similar to that observed with the prediction from HLM data (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication). The activity-based approach proved to be a better scaling estimate than both HLM and P450 abundance, since the predicted clearance was within the 2-fold in vivo range for midazolam, triazolam, diazepam, and alprazolam, although flunitrazepam clearance was under-predicted (Fig. 6). Accurate quantitative estimates of the CYP3A expression levels are highly dependent on the antibody and protein-standards used (Hustert et al., 2001). The considerable variation observed in the amount of nonfunctional apoprotein and the inability of Western blotting technique to distinguish between the active and nonactive P450 may result in the underestimation of P450 abundance and clearance prediction via this approach.
The concentration of accessory proteins (e.g., NADPH P450-oxidoreductase and cytochrome b5) and their relative ratio to the P450 protein can differ considerably between recombinant enzymes and HLM (Venkatakrishnan et al., 2000). CYP3A4 activity can be affected by the lack/addition of these proteins, particularly since cytochrome b5 has been reported to show substrate-dependent stimulatory effect on CYP3A4 activity (Yamazaki et al., 1996; Voice et al., 1999). Nakajima et al. (2002) have reported that differences observed in the NADPH P450-oxidoreductase/P450 ratio in the expression systems were not a critical factor for the quantitative clearance prediction. Based on Hirota et al. (2001) data, RAF estimates ± cytochrome b5 (0.14 and 0.68, respectively) for alprazolam 4-hydroxylation were obtained and applied for the prediction of midazolam CLint in vivo. Predictions observed were in good agreement with the in vivo value for midazolam (422 ± 280 ml/min/kg) (H. C. Rawden, D. J. Carlile, A. Tindall, D. Hallifax, A. Galetin, K. Ito, and J. B. Houston, manuscript submitted for publication) inasmuch as RAF differences counterbalanced the difference in clearance obtained in recombinants ± cytochrome b5. Therefore, estimation of the RAF value and clearance prediction of test compounds are valid if determined under the same conditions (either ±cytochrome b5).
The contribution of CYP2C19 to the overall predicted clearance was minor in comparison to CYP3A4/5. No information on poor metabolizer/extensive metabolizer status in our liver bank was available; however a 28-fold range in mephenytoin 4′-hydroxylation activity observed in our HL Bank encompasses the mephenytoin S/R ratio documented between poor metabolizer and extensive metabolizer subjects (Bertilsson et al., 1989).
Interindividual Variability. CYP3A4 interindividual variability reflects the combined effects of modulation by endogenous compounds, various drugs, and other environmental as well as genetic factors (Thummel and Wilkinson, 1998; Ozdemir et al., 2000). CYP3A4 activity follows unimodal population distribution in contrast to genetic polymorphism seen with CYP2C19 (Wedlund, 2000), since most of the single nucleotide polymorphisms show a frequency of <1 to 2% (Lamba et al., 2002). In contrast, polymorphic expression of CYP3A5 gene (CYP3A5*3, CYP3A5*6, or CYP3A5*7) (Hustert et al., 2001; Floyd et al., 2003) contributes significantly to variable abundance and activity of this enzyme in both liver and small intestine (Kuehl et al., 2001; Lin et al., 2002; Xie et al., 2004).
High variability in relative abundance of CYP3As (73.2 ± 78.2 and 2.1 ± 1.7 pmol of P450/mg protein for CYP3A4 and CYP3A5, respectively) and in their activity assessed by RAF (coefficient of variation = 71 and 92% for CYP3A4 and CYP3A5, respectively) in our HL Bank was an issue of concern in the prediction of clearance from rP450. To overcome the sample size limitations (n = 12) and possible bias in the quantitative prediction of in vivo clearance, bootstrap analysis (1000 simulations) was performed to justify any distribution assumptions in the generated values for the predicted clearance.
Our study indicates a significant contribution of CYP3A5 when present in equivalent amounts to CYP3A4; however, due to low hepatic relative abundance in our liver data set (0.5-6.15 pmol of P450/mg protein), its in vivo significance is probably minor, as shown in a number of studies (Shih and Huang, 2002; Floyd et al., 2003; Westlind-Johnsson et al., 2003). Recent characterization of CYP3A5 genotype and phenotype in a large panel of livers and small intestines by Lin et al. (2002) revealed that CYP3A5 protein content accounted for 31% of the variability in hepatic 1′-hydroxylation of midazolam and a better correlation between total midazolam hydroxylation activity and CYP3A content when contribution of CYP3A5 was included. A similar tendency was observed in our data: incorporation of CYP3A5 relative abundance improved the clearance prediction for midazolam by 13%. Also, a 3-fold increase in the predicted clearance for midazolam was observed when CYP3A5 distribution is assumed to be in the higher range (e.g., up to 50%, Lin et al., 2002; data not shown). Therefore, the CYP3A5 population distribution in liver and gut, and the ability to accurately distinguish CYP3A5 from CYP3A4, from the activity and abundance point of view, needs to be evaluated to make the final decision on the contribution of this enzyme to the overall clearance.
In conclusion, this study indicates a more accurate in vivo clearance prediction from recombinant enzymes by using the RAF approach rather than the relative abundance. In addition, the ability to incorporate the interindividual variability of the corresponding P450s was demonstrated. However, further refinement will be necessary to encompass the CYP3A population distribution both in liver and intestinal abundance and activity, as this information becomes available.
Appendix
In the case of autoactivation, clearance is defined by the following equation [derived from the two-site model (Houston et al., 2003)], assuming that β = 2 [Vmax is equivalent to 2Kp[E]t, where [E]t is the total enzyme concentration (Segel, 1975)].  Similar principles used for the derivation of CLmax from the Hill equation (Houston and Kenworthy, 2000) were applied here, whereas the first derivative, d v/S/d S gives the slope of the clearance plot (eq. 6):
Similar principles used for the derivation of CLmax from the Hill equation (Houston and Kenworthy, 2000) were applied here, whereas the first derivative, d v/S/d S gives the slope of the clearance plot (eq. 6): 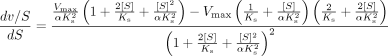
To calculate the x and y coordinates for the inflection point (CLmax), the slope (d v/S/d S) is set to zero and the following equation is obtained: 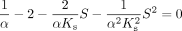
where S is defined as 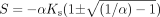
Solving for S under the condition that (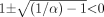 in eq. 8
in eq. 8 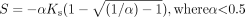
After substitution of the S value into eq. 6, the y value (v/S) of the inflection point is obtained: 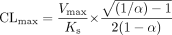
Acknowledgments
We thank Dr. Leon Aarons and Kayode Ogungbenro for advice on bootstrap analysis and Drs. Julie Andrews and Elena Paskaleva for work in estimating CYP3A relative abundance in the liver bank.
Footnotes
-
Financial support for this project was provided by the following Centre for Applied Pharmacokinetic Research Consortium members: Bristol Myers-Squibb, GlaxoSmithKline, Novartis, Pfizer, Roche, and Servier.
-
doi:10.1124/dmd.104.000844.
-
ABBREVIATIONS: rP450, recombinant cytochrome P450; P450, cytochrome P450; CLint, intrinsic clearance; CLmax, maximum clearance; HL, human liver; HLM, human liver microsome(s); RAF, relative activity factor.
- Received June 7, 2004.
- Accepted August 31, 2004.
- The American Society for Pharmacology and Experimental Therapeutics