Abstract
We previously reported that hepatobiliary transporter multidrug resistance-associated protein (MRP2/Mrp2) is considered to be the major cause of the interspecies differences detected by efflux of fluorescent substrates in isolated hepatocytes. In the present study, the interspecies differences of MRP2/Mrp2 were first evaluated by quantitative real-time polymerase chain reaction and Western blotting. The mRNA levels were able to distinguish the difference among species with a rank order comparable with the corresponding activities observed, whereas the extents of the differences remained unknown. The cross-reactions of MRP2/Mrp2 protein of different species with anti-human MRP2 polyclonal antibody were found by Western blotting. However, because of the unknown binding affinity of antibody to MRP2/Mrp2 protein across species and lack of purified MRP2/Mrp2 proteins for calibration, the immunoblotting assay was excluded from the absolute quantification of MRP2/Mrp2 protein for multiple species. By using our newly developed liquid chromatography-tandem mass spectrometry quantification method, we were able to measure the absolute amount of MRP2/Mrp2 in liver tissues and isolated hepatocytes across species. Freshly isolated hepatocytes conserved MRP2/Mrp2 protein levels that are comparable with those in the liver tissues. The amount of Mrp2 in rat liver was approximately 10-fold higher than that in other species. Moreover, a significant loss of Mrp2 protein in the membrane fraction of rat cryopreserved hepatocytes was observed. Thus, the absolute differences of MRP2/Mrp2 levels in various species were determined, for the first time, by direct quantification. The results could potentially fill the translational gaps of in vitro/in vivo or preclinical species to human extrapolation of hepatobiliary elimination mediated by MRP2/Mrp2.
Xenobiotics and their metabolites are generally eliminated and detoxified by phase I and phase II enzymatic metabolism, by phase III transporter-mediated drug efflux to bile, or by both mechanisms. The excretion of drugs by hepatocytes into bile is one of the primary elimination routes for xenobiotics and the conjugate metabolites (Arias et al., 1993). In each stage of drug discovery, accurate prediction of human pharmacokinetics for a potential drug candidate is of great value (Mahmood, 1999). Even though the interspecies scaling methods based on physiologically allometric procedures have been successfully applied, particularly into extrapolation of hepatic enzymatic metabolism and urinary excretion (Dedrick et al., 1970; Iwatsubo et al., 1997; Ito et al., 1998), the in vitro or in vivo model for biliary excretion predication is far from being mature (Mahmood and Sahajwalla, 2002). The remarkable interspecies differences in biliary excretion of xenobiotics and drugs/metabolites (Ishizuka et al., 1999; Shilling et al., 2006) may cause significant overestimation of biliary excretion in humans simply by an exponential allometric extrapolation approach (Lave et al., 1999; Pahlman et al., 1999; Ayrton and Morgan, 2001). Therefore, understanding the molecular mechanisms underlying the marked species difference in hepatobiliary elimination of drugs and their metabolites should greatly advance the current allometric scaling models for estimation of human pharmacokinetics.
Several ATP-binding cassette efflux transporters are responsible for the hepatobiliary elimination of therapeutic agents and physiological substances (Keppler and Arias, 1997; Muller and Jansen, 1997; Suzuki and Sugiyama, 1999). Even though the interspecies differences in other transporters involving hepatobiliary elimination remain obscure, the marked species differences in MRP2/Mrp2 transporter activity have been reported in both in vitro and in vivo models (Ishizuka et al., 1999; Ninomiya et al., 2005; Shilling et al., 2006). For example, Ishizuka et al. (1999) found that the in vitro transport of 2,4-dinitrophenyl-S-glutathione into canalicular membrane vesicles was 8-fold higher (Vmax/Km: 64.2 versus 7.7) in rat than in dog, whereas the in vivo biliary excretion of temocaprilat was 40-fold higher in rat than in dog. Even though species differences in Mrp2-mediated biliary efflux have previously been reported, agreement on the degree of difference remains to be determined, because the absolute differences of transporters in these models remain unknown.
A LC-MS/MS-based absolute quantitative method (also known as AQUA), has been widely used in protein quantification in biological and clinical samples (John et al., 2004). However, the application for the measurement of membrane proteins has been delayed because of their hydrophobic nature and extremely low expression level (Barnidge et al., 2003). In a recent study, a strategy to absolutely quantify MRP2/Mrp2 across species has been developed in our laboratory and has been found to be a highly sensitive, selective, accurate, and precise method (Li et al., 2008b). In the present study, the absolute amount of MRP2/Mrp2 was measured in membrane fractions of liver tissues and isolated hepatocytes from various species. To our knowledge, coupled with the quantitative measurement of mRNA levels and immunologic based protein detection, this was the first comprehensive report of the absolute differences of MRP2/Mrp2 protein levels across species. The findings could provide fundamental support for in vitro/in vivo correlation and preclinical animal to human pharmacokinetics prediction and further promote the progression of pharmaceutical practice in drug discovery.
Materials and Methods
Chemicals and Reagents. High-performance liquid chromatography-grade acetonitrile and water were purchased from Burdick & Jackson (Muskegon, MI) and EMD Chemicals, Inc. (Gibbstown, NJ), respectively. Formic acid and anti-human MRP2 polyclonal antibody were obtained from Sigma-Aldrich (St. Louis, MO). The protein quantification BCA kit and the in-solution digestion kit were purchased from Pierce Biotechnology (Rockford, IL). The ProteoExtract Native Membrane Protein Extraction Kit was purchased from Calbiochem (Temecula, CA). Precast Tris-HCl SDS-polyacrylamide gel electrophoresis gradient gel, Coomassie Blue R-250, and the destaining buffer were purchased from Bio-Rad (Hercules, CA). The enhanced chemiluminescence ECL plus kit was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK). The RNeasy and RNase-free DNase kits were purchased from QIAGEN (Valencia, CA). SYBR SuperMix was purchased from Applied Biosystems (Foster City, CA).
Snap-Frozen Liver Tissues. Snap-frozen liver tissues from 15 normal human donors aged from 1 to 78 years (7 males and 8 females), 6 beagle dogs, 4 rhesus monkeys, and 7 cynomolgus monkeys were obtained from the Pfizer Tissue Bank. Rat livers were isolated from 5 Sprague-Dawley rats obtained from Charles River Laboratories, Inc. (Wilmington, MA). All procedures were approved by the St. Louis Pfizer Institutional Animal Care and Use Committee. The animal care and use program is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Preparation of Cryopreserved and Freshly Isolated Hepatocyte Suspension. Cryopreserved hepatocytes were from Pfizer central hepatocyte stock (In Vitro Technologies, Baltimore, MD). The lot numbers of cryopreserved hepatocytes used in this study were as follows: IOE, PXK, and OZL for human; FGJ, MON, and GWJ for Sprague-Dawley rat; FPA and EYS for beagle dog; and EHH and FAM for monkey. The hepatocyte isolation kits were purchased from XenoTech, LLC (Kansas City, KS). Frozen cryopreserved hepatocytes were thawed according to the vendor's instructions. In brief, hepatocytes were thawed at 37°C in a shaking water bath for 1.5 min and immediately poured into tube A of the kit (supplemented Dulbecco's modified Eagle's medium containing Percoll). The tube was then centrifuged for 3 min at 85 to 90g for human and monkey hepatocytes or 70 to 75g for rat and dog hepatocytes. The supernatant was removed, and the hepatocyte pellet was then resuspended with 5 ml of medium of tube B of the kit to check the cell viability. Cell viability was assessed by a trypan blue exclusion method. Hepatocytes were then washed with the washing buffer from the membrane extraction kit and applied for membrane fraction extraction.
Freshly isolated hepatocytes were purchased from CellzDirect (Pittsboro, NC). The lot numbers of fresh hepatocytes were as follows: Hu707, Hu780, and Hu0731 (human); Rs354 and Rs416 (rat); Db176 and Db167 (dog), and Cy215, Cy230, and Cy236 (monkey). Upon arrival, the fresh hepatocytes were centrifuged at 4°C for 4 min under the same conditions as described above. The supernatant was removed, and the cell pellet was resuspended with 5 ml of medium to check the cell viability with the trypan blue exclusion method. The hepatocytes were then washed with washing buffer from the membrane extraction kit, and the membrane fraction was extracted using the same procedure as described below. Fresh and cryopreserved hepatocytes with viability greater than 80% were used.
RNA Extraction and Quantitative RT-PCR. Frozen liver tissues were ground to a fine powder in liquid nitrogen and weighed before processing. For cryopreserved hepatocytes and fresh isolated hepatocytes, RNA was extracted after Percoll preparation as described above. The isolated RNA was treated with DNase to digest genomic DNA and quantified with a nano-UV-spectrometer (NanoDrop Technology, Wilmington, DE). The first-strand cDNA was prepared from 200 ng of total RNA by using the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA) with random hexamer primers according to the manufacturer's instruction. Negative controls were prepared without the presence of reverse transcriptase. All quantitative PCR reactions were prepared using SYBR PCR SuperMix with the synthesized first-strand cDNA and specific primers and performed using an ABI-PRISM 7500 Fast Detection System (Applied Biosystems). The thermal cycling condition comprised 10 min at 95°C and 40 cycles alternating at 95°C for 30 s, denaturing at 58°C for 30 s, and extending at 60°C for 30 s. Quantification of gene expression was performed using the 2–ΔΔCt approach to calculate the relative changes normalized to housekeeping gene GAPDH.
Extraction of Membrane Fraction. The membrane protein fraction was extracted using the Native Membrane Protein Extraction kit (EMD Biosciences, Inc., Darmstadt, Germany) according to the manufacturer's suggested protocol. In brief, the crushed liver tissues and hepatocyte pellets were homogenized in extraction buffer I containing the appropriate amount of protease inhibitor cocktail after incubation at 4°C for 10 min while rotating. The homogenate was centrifuged at 14,000 rpm for 15 min at 4°C. The supernatant containing cytosolic protein was removed, and the pellets were resuspended in extraction buffer II with the proper amount of protease inhibitors. After 30 min of incubation at 4°C with rotating, the suspension was centrifuged at 14,000 rpm for 15 min at 4°C. The supernatant-containing membrane protein was collected and stored at –80°C for future analysis. Protein concentrations of the membrane fraction obtained were determined with a BCA protein assay kit (Pierce Biotechnology).
Western Blotting Analysis. An aliquot of the membrane extraction (30 μg of protein) was mixed with an equal volume of Laemmli buffer (Bio-Rad) and incubated at room temperature for 5 min. The denatured samples were then fractionated on 4 to 20% gradient SDS gel (Bio-Rad) and electrophoretically transferred on to a nitrocellulose membrane (Bio-Rad). After incubation with blocking reagent, the membrane was washed and incubated with anti-human MRP2 polyclonal antibody at 1:500 dilution overnight at 4°C. Bound antibody was detected with horseradish peroxidase-conjugated anti-rabbit antibody and visualized with the enhanced chemiluminescence ECL plus kit. Coomassie Blue staining of the gel served as a loading control.
Tryptic Digestion and Sample Preparation. The membrane protein samples were diluted to a working concentration of 2 μg/μl, and 20 μlofthe extracted membrane protein (40 μg of protein) was subsequently reduced with 10 mM dithiothreitol and alkylated with iodoacetamide in 50 mM ammonium bicarbonate digestion buffer. After addition of 50 fmol of stable isotope-labeled (SIL) MRP2 peptide serving as the internal standard (Li et al., 2008b), the protein samples were digested by trypsin in a final volume of 40 μl at 37°C for 4 h and then at 30°C for 14 h. The optimal ratio of trypsin and protein was 1:50. At the end of digestion, the samples were acidified with equal amounts of 50% acetonitrile in H2O containing 0.1% formic acid and centrifuged at 3000 rpm for 20 min before LC-MS/MS analysis. A 16-mer synthetic peptide corresponding to MRP2/Mrp2 tryptic fragment (LTIIPQDPILFSGSLR) and the SIL internal standard (LTIIPQDPILFSGSL[13C615N1]R) were obtained from Celtek Bioscience (Nashville, TN) and Sigma-Aldrich, respectively. The calibration curve was prepared at a range of concentrations of the synthetic AQUA peptide, respectively, of 31.25, 62.5, 125, 250, 500, 1000, and 2500 pM with the SIL internal standard at a fixed concentration of 2500 pM. Data were processed by integrating the appropriate peak areas generated from the reconstructed ion chromatograms for the 16-mer analyte peptide and the SIL internal standard peptide by Analyst 1.4.1 (Applied Biosystems). The ratio of the peak area of the AQUA peptide to the SIL peptide (y) was plotted against the concentration of the synthetic native peptide (x) for constructing the regression analysis.
LC-MS/MS Quantitative Measurement of MRP2/Mrp2 Protein. Sample quantification was conducted by coupling a triple quadruple mass spectrometer (API 4000; Applied Biosystems) to a Shimadzu LC (SLC-10A) system (Shimadzu, Wooldale, IL) and HTS PAL Leap Autosampler (Leap Technologies, Carrboro, NC). The LC column used for peptide separation and elution was a 2.1 × 100 mm C18 column containing 5-μm size beads with 300 Å pore size (Vydac EVEREST). The mobile phase A was water with 0.1% v/v formic acid, whereas mobile phase B was acetonitrile with 0.1% v/v formic acid. A linear gradient was used to achieve the chromatographic separation starting from 5% B and progressing to 35% B over a period of 30 min. A sample volume of 20 μl was injected onto the LC column at a flow rate of 0.4 ml/min. The parent-to-product transition for the AQUA peptide monitored represented the doubly charged parent ion (LTIIPQDPILFSGSLR)2H+ (m/z 886.0) to the single charged product y ion with m/z 1331.6. Likewise, the transition selected for the SIL internal standard peptide is the analogous doubly charged parent ion detected with m/z 889.6 to the single charged product y ion with m/z 1338.6. The instrument settings of the API4000 triple quadruple mass spectrometer were as follows: ion spray voltage, 4 kV; temperature, 400°C; declustering potential, 50 V; collision energy, 38 V; entrance potential, 10 V; and collision cell exit potential, 11V.
Data Analysis. Data are representative of a minimum of two experiments performed on different days. The MRP2/Mrp2 protein amount across species was statistically analyzed using one-way ANOVA. Comparison of MRP2/Mrp2 expression between isolated hepatocytes and frozen liver tissues or between freshly isolated and cryopreserved hepatocytes of a single species was statistically analyzed using Student's t test. A value p < 0.05 was regarded as statistically significant.
Results
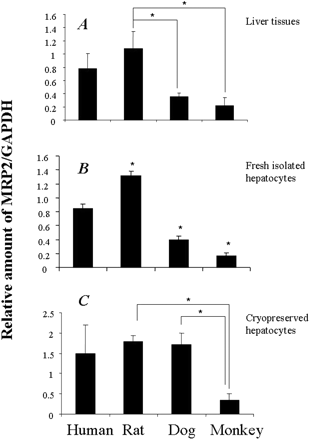
Quantitative mRNA detection as a surrogate protein measurement has been very popular for investigation of the differences in transporter protein levels in in vitro or in vivo models (Goh et al., 2002; Hilgendorf et al., 2007). Approaching on that front, we examined the mRNA level in liver tissues and isolated hepatocytes by using qRT-PCR. Table 1 lists the primers for MRP2/Mrp2 and the control housekeeping gene GAPDH of four tested species. In freshly isolated hepatocytes and livers, the highest amount of Mrp2 mRNA among the tested species was expressed in rats, whereas the least was expressed in monkey. The mRNA level ranked as rat > human > dog > monkey (p < 0.05 by ANOVA) (Fig. 1, A and B). In cryopreserved hepatocytes, the mRNA level of Mrp2 in monkey was the lowest among the tested species, whereas no significant differences were found among human, rat, and dog (Fig. 1C).
Human, rat, dog, and monkey MRP2/Mrp2 and housekeeping gene qRT-PCR primers
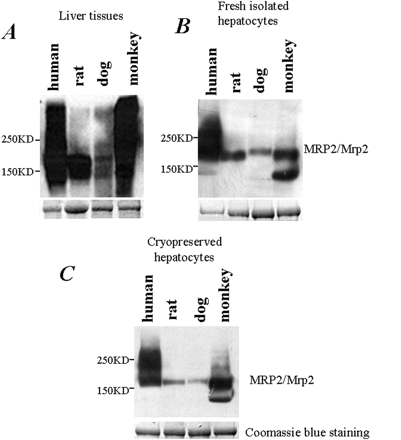
Immunoblotting-based protein detection (e.g., Western blotting) has been a widely used assay for protein detection and quantification for decades. By using the commercially available anti-human MRP2 polyclonal antibody, we performed Western blotting assay to detect the MRP2/Mrp2 protein in membrane extractions across species. As shown in Fig. 2, anti-human polyclonal antibody was cross-reacted with the MRP2/Mrp2 proteins extracted from liver tissue (Fig. 2A), freshly isolated (Fig. 2B), or cryopreserved hepatocytes (Fig. 2C) of various tested species. Apparently, the MRP2/Mrp2 protein of human and monkey exhibited the highest cross-reactivity with the antibody, whereas the dog exhibited the least. However, because of the lack of the purified MRP2/Mrp2 proteins as calibration standard for each species, we were not able to compare the protein level across species. In addition, the results were not consistent with the mRNA levels of MRP2/Mrp2 and also not in the agreement with the differential transport activities of MRP2/Mrp2 reported previously (Ishizuka et al., 1999; Mahmood and Sahajwalla, 2002; Shilling et al., 2006; Li et al., 2008a).
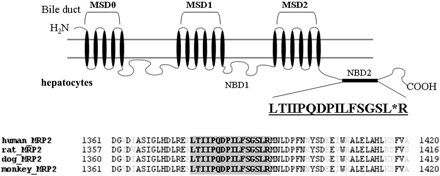
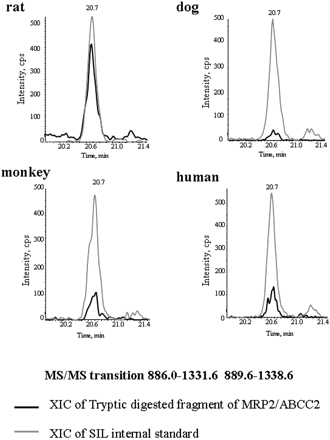
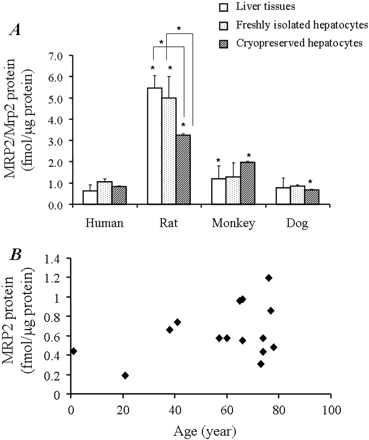
In a recent study, a LC-MS/MS-mediated MRP2/Mrp2 protein quantification method has been developed in our laboratory. The method has been validated as a highly sensitive and selective quantitative approach with great accuracy and precision (Li et al., 2008b). In the present study, the LC-MS/MS method was applied to absolutely measure MRP2/Mrp2 protein in membrane fraction extracted from freshly isolated/cryopreserved hepatocytes and frozen liver samples across species. The strategy that we used was to select the proteotypic peptide representing MRP2/Mrp2 protein. The process includes a combination of comprehensive MS/MS verification of the candidate peptides and in silico prediction of the tryptic digested fragments by using the online software http://prospector.ucsf.edu/. To obtain the selective peptide exclusively representing MRP2/Mrp2 across species, a further genome-wide BLAST search was conducted to ensure the specificity. Figure 3 shows the alignment of the MRP2/Mrp2 sequence across species and highlights the position of the proteotypic peptide. A synthetic peptide (LTIIPQDPILFSGSLR) representing the fragment cleaved from tryptic proteolysis of MRP2/Mrp2 protein serves as the calibration standard for LC-MS/MS quantification. Figure 4 exhibits the examples of reconstituted ion chromatograms representing the MRP2/Mrp2 peptide produced by tryptic digestion of liver membrane proteins from various species and the internal standard, SIL peptide added before tryptic digestions. A quality control study was performed to ensure the performance of the MRP2/Mrp2 quantitative method by LC-MS/MS. Both the accuracy (relative error) and precision (coefficient of variation) of all quality control results were less than 15% (Table 2), at two known concentrations (0.625 and 1.64 nM) of synthetic AQUA peptide spiked in the biological matrix prepared from liver samples. In addition, the great recovery rate also indicated that the potential variations caused by the discrepancy of biological matrix from different species could be omitted (Table 2). In the present study, snap-frozen liver tissues of 15 human donors aged from 1 to 78 years old, 5 rats, 6 dogs, and 11 monkeys were subjected to LC-MS/MS quantitative analysis. MRP2/Mrp2 protein measurement was also conducted for the pooled samples from three lots of cryopreserved hepatocytes of human and rats and two lots for dog and monkey. The absolute amount of MRP2/Mrp2 protein in freshly isolated hepatocytes was averaged from the lots prepared from three individual donors for human and monkey and two donors for rat and dog. Figure 5A summarizes the quantitative results of MRP2/Mrp2 in liver tissues and freshly isolated and cryopreserved hepatocytes of human, rat, dog, and monkey. In liver tissue, the MRP2/Mrp2 protein level ranked rat ≫ monkey > dog ≈ human with the average ranging from 0.6 to 5.5 fmol/μg of membrane protein. A comparable amount of MRP2/Mrp2 was detected in freshly isolated hepatocytes of all tested species. The absolute amount of Mrp2 protein in rat was 10-fold higher in liver tissue than that in human. Impressively, a greater variation of MRP2 expression was observed in human liver donors (6-fold, ranging from 0.2 to 1.2 fmol/μg protein) and the animals (monkey and dog, ∼4-fold, ranging from 0.6 to 2.7 and 0.5 to 1.7 fmol/μg protein, parallel, respectively) that have been tested with discovery compounds before tissue collecting, compared with less than 2-fold variation in rat (ranged from 4.6 to 6.1 fmol/μg protein).
Quality control of synthetic proteotypic peptide in membrane protein matrix across species
Comparison of mRNA levels of MRP2 in isolated hepatocytes and liver tissue across species. The mRNA level of MRP2/Mrp2 was normalized to GAPDH. The data represent the relative fold of mRNA level of Mrp2 gene to one of human subjects (mean ± S.D.; n = 3 or 4). The panels from top to bottom represent liver tissues (A), freshly isolated hepatocytes (B), and cryopreserved hepatocytes (C). One-way ANOVA analysis indicated that the difference in mRNA levels of MRP2 among the four species tested was not statistically significant in cryopreserved hepatocytes and was statistically significant in fresh isolated hepatocytes and liver tissues (p < 0.05). The paired comparison among the species tested was performed with Student's t test. In liver tissues and cryopreserved hepatocytes, the statistically significant pairs are highlighted as * (p < 0.05). In fresh cryopreserved hepatocytes, the difference between human and any other tested species was statistically significant.
Evaluation of MRP2/Mrp2 protein levels across species via Western blotting. A 30-μg membrane protein fraction prepared from snap-frozen liver tissues (A), freshly isolated hepatocytes (B), and cryopreserved hepatocytes (C) of four different species was separated on SDS-polyacrylamide gel electrophoresis. The MRP2/Mrp2 protein was detected by using anti-human MRP2 polyclonal antibody. In the bottom panels of A, B, and C, Coomassie Blue staining served as the loading control.
Discussion
MRP2/ABCC2, belonging to the ATP-binding cassette transporter superfamily, is one of the major efflux transporters localized on the hepatic canalicular membrane in liver and has been demonstrated to be responsible for interspecies difference in hepatobiliary secretion (Niinuma et al., 1997; Ishizuka et al., 1999; Shitara et al., 2005). Species differences in MRP2/Mrp2 activity have been reported in both in vivo and in vitro models (Ishizuka et al., 1999; Shilling et al., 2006) and are considered to be one of the major causes of the failure in interspecies scaling from preclinical animal to human. As a general rule, two major factors are commonly used to characterize transporter-mediated drug clearance: Vmax and Km. Although the Km is the unique property of a certain transporter substrate and usually is a fixed parameter, Vmax is determined by the expression level of a given transporter in a tissue or the model being applied. The Vmax varies by tissues and individual species and is sensitive to treatment with inducers and suppressors. During the past decade, various in vitro systems have been developed to determine the transport kinetics or modulate recognition in a variety of species. However, direct comparisons of the absolute amount of MRP2/Mrp2 protein across species have not been addressed. In addition, as the in vitro “golden tool” for drug metabolism research, freshly isolated and cryopreserved hepatocytes are most commonly and widely used as in vitro liver models in drug discovery. The retention of hepatobiliary transporters remains unknown, when the polarized structure of hepatocytes is disrupted during the hepatocyte isolation/cryopreservation process. In a recent study, we demonstrated that the elimination rate of an MRP2/Mrp2-specific substrate was 4- to 6-fold faster in isolated rat hepatocytes than in human hepatocytes (Li et al., 2008a). However, the extrapolation of transporter-mediated drug clearance from in vitro to in vivo still remains a challenge because the assumption was made that the amount of transporters remains unchanged or underwent a similar degree of loss during the hepatocyte preparation from each species. As another example, the internalization of Mrp2 and P-glycoprotein in freshly isolated rat hepatocytes has recently been reported (Bow et al., 2008). The report could be expected to produce obvious discrepancies in the functional efflux observed in isolated hepatocytes (Oude Elferink et al., 1990; Lam and Benet, 2004; Li et al., 2008a). Therefore, investigation into the absolute amount of transporter proteins existing in isolated or cultured hepatocytes would be of great value for understanding the protein-activities relationship.
Schematic representation of the membrane topology of MRP2/Mrp2 and protein alignment across species. The selected AQUA peptide is highlighted in gray. The SIL internal standard is indicated with a single residue substitution of [13C615N1]Leu. GenBank numbers: human MRP2, NP_000383; rat Mrp2, NP_036965; dog Mrp2, NP_001003081; monkey Mrp2, NP_001028019.
Several attempts were made in our laboratory to elucidate the molecular mechanisms underlying the species differences of MRP2/Mrp2 activities in isolated hepatocytes (Li et al., 2008a). As mRNA quantification has been used widely as an alternate measurement of protein, we first evaluated the species difference of MRP2/Mrp2 in mRNA expression. In both liver tissues and isolated hepatocytes, the rank order and the extent of difference in mRNA levels among the species were not consistent with the corresponding activities reported previously (Li et al., 2008a) or the uptake Vmax of MRP2 substrate, 2,4-dinitrophenyl-S-glutathione, into canalicular membrane vesicles prepared from rat and human liver (1.9 versus 0.23 nmol/min/mg protein) (Ishizuka et al., 1999). The results revealed that the mRNA levels of MRP2/Mrp2 in liver tissue or isolated hepatocytes might not directly reflect the amount of functional protein existing on plasma membrane. In fact, despite some examples of the concordance between mRNA and protein expression of the drug transporters published in literature, skepticism regarding mRNA as a surrogate protein measurement is frequently a concern (Behrens et al., 2004; Taipalensuu et al., 2004; Jones et al., 2005). It has been reported that the translation of rat Mrp2 mRNA is differentially regulated by the upstream open reading frames in the 5′-untranslated region (Zhang et al., 2007) and results in the disconnection between mRNA level and protein amounts. Furthermore, post-transcriptional mechanisms may play a more prominent role in lipopolysaccharide-induced regulation of human MRP2 and bile salt export pump compared with the counterparts in rat (Elferink et al., 2004). Thus, one must exercise caution with regard to using qRT-PCR as an alternative approach to absolute quantification of MRP2/Mrp2 protein in liver or isolated hepatocytes. The different mRNA expression profile observed in cryopreserved hepatocytes (both in individual measurements and rank order) compared with that in liver tissues and fresh isolated hepatocytes was postulated to result from the cryopreservation procedure, which was further continued by the absolute measurement of MRP2/Mrp2 protein discussed below. Because of the discrepancy between the mRNA expression level and the corresponding transport activities of MRP2/Mrp2, a more relevant characterization, i.e., direct quantification, of interspecies differences in hepatobiliary transporters should be considered. Therefore, in the present study, we also attempted to quantify the proteins by immunoblotting assay. However, given the unknown affinity of this antibody to MRP2/Mrp2 protein of different species, purified MRP2/Mrp2 proteins are essential as the calibration standard for protein quantification. In this context, the immunoblotting-based approach for protein quantification of MRP2/Mrp2 was excluded owing to lack of the purified proteins.
Coupled to one- or two-dimensional gel electrophoresis, LC-MS/MS has been increasingly used for a variety of biomarker evaluations and quantification (Wu et al., 2002; Barnidge et al., 2004; Kuhn et al., 2004). In a recent study, we have overcome serial obstacles in developing a method to absolutely quantify MRP2/ABCC2 for multiple species by LC-MS/MS (Li et al., 2008b). In the present study, we were able to demonstrate the absolute amount of MRP2/Mrp2 protein ranked as rat ≫ monkey > human ≈ dog in liver tissues and isolated hepatocytes. Although the level of MRP2/Mrp2 protein in the membrane fraction of freshly isolated hepatocytes was conserved comparable with that of whole liver tissue, a significant loss (40%) of Mrp2 was found in rat cryopreserved hepatocytes at 3.24 versus 5.71 or 5.45 fmol/μg in fresh isolated hepatocytes and frozen liver tissues, respectively (Fig. 5A). Even though the causes of the Mrp2 decrease in rat cryopreserved hepatocytes were not explored here, it is speculated that the cryopreservation process might alter the membrane density of Mrp2 protein. Significantly, the remarkable difference in Mrp2 protein level between rat cryopreserved and freshly isolated hepatocytes correlated with the previous finding that the elimination half-life of glutathione conjugates of 5-chloromethylfluorescein diacetate (a MRP2/Mrp2 substrate) was significantly longer in rat cryopreserved hepatocytes than in the freshly isolated counterpart (Li et al., 2008a). Under the notion that there could be absolute differences of MRP2/Mrp2 in liver tissues, Shilling et al. (2006) addressed the species difference of uptake clearance of leukotriene C4 (a MRP2/Mrp2 substrate) in canalicular membrane vesicles among different species. The results suggest that the absolute amount of MRP2/Mrp2 could serve as the key in the biliary excretion activities.
Representative reconstituted ion chromatogram of LC-MS/MS analysis of MRP2/Mrp2 peptide released from tryptic digestion in liver tissues of rat, dog, monkey, and human are shown.
In the present study, a larger variation of the amount of MRP2 was observed in human samples However, MRP2 protein level in livers among human donors varies in an age-independent manner (Fig. 5B), and there was also no significant difference between male and female (data not shown). Previous studies showed that multiple mechanisms underlie the regulation of MRP2/Mrp2 expression. For example, the MRP2 level in human placenta was affected by gestational age with an increased MRP2 protein level at later stages of pregnancy (Meyer zu Schwabedissen et al., 2005). In rat, Mrp2 protein started to be detected in livers of 16- and 20-day-old fetuses and tend to increase gradually after birth (Zinchuk et al., 2002). Administration of xenobiotics, including cycloheximidine, 2-acetylaminofluorene, and rifampin has been shown to influence on the expression level of Mrp2 protein (Buchler et al., 1996; Fromm et al., 2000; Courtois et al., 2002). Moreover, different endogenous molecules are considered to be involve in the regulation of Mrp2 expression (Hartmann et al., 2002). Nevertheless, whether environmental and/or genetic factors affect MRP2/Mrp2 expression still remains largely unknown. Without donor information regarding medication history or disease status, we are as yet unable to explain the MRP2 variation within the human liver samples used in our study.
Absolute quantification of MRP2/Mrp2 protein levels in isolated hepatocytes and liver tissues from different species. A, membrane protein fraction prepared from pooled cryopreserved hepatocytes from two to three lots; a single lot of fresh isolated hepatocytes; and frozen liver tissues of 15 human donors, 5 rats, 6 dogs, and 11 monkeys were subjected to selected reaction monitoring analysis for absolute quantification of MRP2/Mrp2 protein. The data represent mean ± S.D. One-way ANOVA analysis indicated that the MRP2/Mrp2 protein levels among the four species tested are statistically significant (p ≤ 0.05). Student's t test was used to compare the differences in MRP2/Mrp2 protein levels in human and other tested species and the differences in Mrp2 level among rat cryopreserved hepatocytes and fresh hepatocytes and liver tissues. *, mean p ≤ 0.05. B, correlation between the amount of MRP2 protein and the age of the individual donor.
It is pertinent to note that the difference in the protein levels of MRP2/Mrp2 measured does not necessarily reflect the difference in transport activity reported in animal models and in in vitro systems. For instance, the rank order of the amount of MRP2/Mrp2 protein in livers was rat ≫ monkey > dog ≈ human. The difference in protein levels here matched with the respective glutathione conjugates of 5-chloromethylfluorescein diacetate and calcein efflux rate in isolated hepatocytes reported previously (Li et al., 2008a), except that a shorter elimination half-life of Mrp2 substrate was observed in dog hepatocytes. The inconsistency of MRP2/Mrp2 protein level with the corresponding transport activities indicates that the difference in MRP2/Mrp2 transporter activity may be ascribed to differential binding affinities of compounds to MRP2/Mrp2 transporters in the different species. In fact, Ninomiya et al. (2006) reported that dog Mrp2 transporter contained both high-affinity binding site with Km values similar to those of rat Mrp2 and an additional low-affinity site, which was the major contributor for some Mrp2 substrates. Moreover, the difference in binding affinity between human MRP2 and rat Mrp2 may be the mechanism underlying the differential excretion ratio of the glucuronized form of mycophenolic acid (a MRP2/Mrp2 substrate) between human and rats (Takekuma et al., 2007). Taken together, these results reveal that the pronounced interspecies difference in the biliary excretion activity of MRP2/Mrp2 substrates can result from the sum of the protein level (Vmax) and the intrinsic transporter affinity (Km) for its substrates (Ishizuka et al., 1999; Ninomiya et al., 2005).
In conclusion, we have systematically investigated the mRNA levels and protein levels of hepatic efflux transporter MRP2/Mrp2 in freshly isolated, cryopreserved hepatocytes and snap-frozen liver tissues from various species. By using the LC-MS/MS AQUA method, we quantitatively measured the absolute amount of MRP2/Mrp2 protein across species, with the order ranking of rat ≫ monkey > dog ≈ human in liver tissue. Coupled with the determination of in vitro transport kinetic parameters (e.g., Km), the absolute comparison of MRP2/Mrp2 protein across species could facilitate the interspecies scaling of pharmacokinetic parameters in drug discovery. Further investigation on protein synthesis and degradation of hepatic efflux transporter MRP2/Mrp2 (functional protein versus nonfunctional protein) will enable improved understanding of the marked differences in biliary excretion across species.
Acknowledgments
We thank Drs. Joseph Fleishaker, Timothy Heath, and Andrew Lickteig for their helpful comments and suggestions on the manuscript. We also thank Frank Voigt from the Pfizer Tissue Bank for preparing the snap-frozen liver tissues for this study and Dean Messing, Steve Wene, and Kathy J. Hotz for preparation of rat, dog, and monkey liver tissues.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.108.023234.
-
ABBREVIATIONS: MRP/Mrp, multidrug resistance-associated protein; LC, liquid chromatography; MS/MS, liquid chromatography tandem mass spectrometry; AQUA, absolute quantification; RT, real-time; PCR, polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SIL, stable isotope labeled; ANOVA, analysis of variance; q, quantitative.
- Received July 2, 2008.
- Accepted October 1, 2008.
- The American Society for Pharmacology and Experimental Therapeutics