Abstract
Cytochromes P450 (P450s) 3A, 2C, and 1A2 constitute the major “pieces” of the human liver P450 “pie” and account, on average, for 40, 25, and 18%, respectively, of total immunoquantified P450s (J Pharmacol Exp Ther 270:414–423, 1994). The P450 profile in the human small intestine has not been fully characterized. Therefore, microsomes prepared from mucosal scrapings from the duodenal/jejunal portion of 31 human donor small intestines were analyzed by Western blot using selective P450 antibodies. P450s 3A4, 2C9, 2C19, and 2J2 were detected in all individuals and ranged from 8.8 to 150, 2.9 to 27, <0.6 to 3.9, and <0.2 to 3.1 pmol/mg, respectively. CYP2D6 was detected in 29 individuals and ranged from <0.2 to 1.4 pmol/mg. CYP3A5 was detected readily in 11 individuals, with a range (average) of 4.9 to 25 (16) pmol/mg that represented from 3 to 50% of total CYP3A (CYP3A4 + CYP3A5) content. CYP1A1 was detected readily in three individuals, with a range (average) of 3.6 to 7.7 (5.6) pmol/mg. P450s 1A2, 2A6, 2B6, 2C8, and 2E1 were not or only faintly detected. As anticipated, average CYP3A content (50 pmol/mg) was the highest. Excluding CYP1A1, the remaining enzymes had the following rank order: 2C9 > 2C19 > 2J2 > 2D6 (8.4, 1.1, 0.9, and 0.5 pmol/mg, respectively). Analysis of a pooled preparation of the 31 donor specimens substantiated these results. In summary, as in the liver, large interindividual variation exists in the expression levels of individual P450s. On average, CYP3A and CYP2C9 represents the major pieces of the intestinal P450 pie, accounting for 80 and 15%, respectively, of total immunoquantified P450s.
The cytochromes P450 constitute a superfamily of heme-thiolate proteins that catalyze the biotransformation of both endo- and xenobiotics, the latter including a myriad of widely prescribed drugs. In humans, approximately 80% of oxidative metabolism and almost 50% of the overall elimination of commonly used drugs can be attributed to one or more of the various P450 enzymes that are classified into three families (CYP1, -2, and -3) (Wilkinson, 2005). The P450s are concentrated prominently in the liver, the principal organ of drug elimination (Lin and Lu, 2001). Therefore, hepatic P450-mediated metabolism represents the major means by which the body eliminates drugs.
A dozen years have passed since the pioneering work by Shimada et al. (1994), who systematically characterized the major P450s involved in the biotransformation of drugs and other xenobiotics. Using microsomes prepared from 60 human donor livers and various anti-P450 antibodies, the CYP3A subfamily (i.e., CYP3A4+CYP3A5) was reported as the most abundant, with a mean specific content that represented 29% of total (spectrally determined) P450 content. The CYP2C subfamily (i.e., CYP2C8+CYP2C9+CYP2C18+CYP2C19) was the second most abundant (18%), followed by CYP1A2 (13%), CYP2E1 (7%), CYP2A6 (4%), CYP2D6 (<2%), and CYP2B6 (<1%). Large interindividual variation was also observed, ranging from 20- (CYP2E1, CYP3A4) to >1000-fold (CYP2D6) (Shimada et al., 1994). Because of the prominent role of the P450s in drug elimination, such variations account in part for the notoriously large interindividual variation observed in human drug clearance and, for some drugs taken per oral, in bioavailability. Thus, in some instances, variation in hepatic P450 expression can contribute to variation in drug response (Wilkinson, 2005).
In addition to the liver, the P450s are expressed appreciably in the small intestinal mucosa, lung, kidney, brain, olfactory mucosa, and skin. Of these tissues, Lin and Lu (2001) surmised that the intestinal mucosa is the most important extrahepatic site of drug biotransformation. As a consequence, the potential exists for substantial presystemic metabolism and thus an enhanced reduction in bioavailability as the drug passes, sequentially, through the small intestine and liver.
As in the liver, CYP3A is the most abundant P450 subfamily expressed in the small intestine, with an average (or median) specific content representing from 50 to 70% of spectrally determined P450 content (Watkins et al., 1987; Paine et al., 1997). Like hepatic CYP3A, enteric CYP3A is localized in a single cell type, specifically, within the mature absorptive columnar epithelial cells (enterocytes) that largely compose the mucosal lining (Kolars et al., 1994). Enteric microsomal CYP3A content, as well as associated catalytic activity, is generally highest in the proximal region and then declines sharply toward the distal ileum (de Waziers et al., 1990; Paine et al., 1997; Zhang et al., 1999). Also in common with hepatic CYP3A, considerable interindividual variation exists in enteric CYP3A, with specific content/activity in the proximal region (i.e., duodenum/proximal jejunum) ranging in values that overlap with those in the liver (Paine et al., 1997).
Although the total mass of CYP3A in the entire small intestine has been estimated to be ∼1% of that in the liver (Paine et al., 1997; Yang et al., 2004), human studies have demonstrated that enteric CYP3A can contribute significantly, and in some cases equally with hepatic CYP3A, to the overall first-pass metabolism of several drugs (e.g., cyclosporine, midazolam, and verapamil) (Kolars et al., 1991; Paine et al., 1996; von Richter et al., 2001). All of these substrates are absorbed by the transcellular route. Therefore, this apparent obligatory passage of drug molecules through the enterocytes, coupled with comparable microsomal CYP3A content/activity between the intestine and liver, may be more important than total enzyme mass in governing the extent of first-pass metabolism in the intestine.
Whereas the abundance and importance of CYP3A in the intestine has been established for some time, relatively less is known about other P450s. Although the CYP2C subfamily has been reported as the second most abundant, the relative contributions of individual members to total P450 content were not ascertained (Klose et al., 1999; Obach et al., 2001; Lapple et al., 2003). Likewise, CYP1A1 (Prueksaritanont et al., 1996; Paine et al., 1999), CYP2D6 (de Waziers et al., 1990; Madani et al., 1999), CYP2J2 (Matsumoto et al., 2002), and CYP3A5 (Lin et al., 2002) have been quantified and demonstrated as catalytically active, but their relative contributions to total intestinal P450 content have not been ascertained. Therefore, we systematically characterized the interindividual variation in the specific contents of these and other P450 enzymes in microsomes prepared from the duodenal/proximal portion of 31 human donor small intestines. Results demonstrated that the P450 profile in the proximal human small intestine is distinct from that in the liver.
Materials and Methods
Materials and Chemicals. Chemicals and reagents for the polyacrylamide gels [sodium dodecyl sulfate (SDS), bis/acrylamide (37.5:1), ammonium persulfate, and N,N,N′,N′-tetramethylethylenediamine] were purchased from Bio-Rad (Hercules, CA). Human lymphoblast-expressed CYP1A1, -2A6, -2B6, -2C8, -2D6*1, -2E1, and -3A4, baculovirus-expressed CYP3A5, and the corresponding primary antibodies (except for CYP2C8) for detection by immunoblot analysis (polyclonal anti-CYP1A1, MAB-2A6, WB-2B6-PEP, polyclonal anti-CYP2D6, polyclonal anti-CYP2E1, WB-3A4, and WB-3A5) were purchased from BD Gentest (Woburn, MA). Baculovirus-expressed CYP2C9*1 and -2J2 and the rabbit polyclonal antibody raised against the CYP2J2-specific peptide HMDQNFGNRPVTPMR (anti-CYP2J2pep1) had been characterized previously (Haining et al., 1996; King et al., 2002). Details of the generation of recombinant CYP2C19 and the anti-CYP2C19 polyclonal antibody immediately follow this section. The secondary antibodies (rabbit anti-goat, rabbit anti-mouse, and goat anti-rabbit horseradish peroxidase-conjugated IgGs) were purchased from Zymed Laboratories (South San Francisco, CA). Polyvinylidene difluoride membranes (Hybond-P) and reagents for enhanced chemiluminescence were purchased from Amersham Biosciences, Inc. (Piscataway, NJ). All other chemicals and reagents were of electrophoresis or analytical grade where appropriate.
Recombinant CYP2C19 and Anti-CYP2C19 Polyclonal Antibody. Recombinant human CYP2C19 was generated using a baculovirus expression system. The cDNA encoding CYP2C19, obtained in the plasmid pBlueScript SK (Stratagene, La Jolla, CA), was a gift from Dr. Joyce Goldstein (National Institute of Environmental Health Sciences, Research Triangle Park, NC) (clone 11a; Romkes et al., 1991). A fragment containing the entire coding frame was subcloned on an XhoI/XbaI restriction endonuclease site into the baculovirus transfer vector pBacPAK8 (Clontech Laboratories, Palo Alto, CA). Cotransfection of this plasmid with Bsu36I-digested Autographica californica nuclear polyhedrosis virus (BacPAK 6; Clontech) into Trichoplusia ni insect cells yielded recombinant virus plaques in which the CYP2C19 gene was placed behind the viral polyhedron promoter. The isolation and amplification of this virus, cell culture methods, the addition of heme cofactor, culture harvesting, and purification of active enzyme were carried out as described previously (Haining et al., 1996).
For the generation of the anti-CYP2C19 polyclonal antibody, recombinant CYP2C19 (8.8 μM) was mixed with complete Freund's adjuvant and injected into a New Zealand White rabbit, followed in 3 weeks by a second booster injection made in incomplete Freund's adjuvant. One week after this booster injection, antibody production was confirmed from a test bleed and dot blot. The crude polyclonal antibody in serum did not cross-react with recombinant CYP2D6. Thereafter, 15 ml of blood was collected at 2-week intervals. The IgG fraction of pooled serum samples was purified using a standard protein-A/agarose column to bind and elute using the Fc portion of the antibody (Maniatis et al., 1989) to yield a 9.9 mg/ml IgG preparation. To boost the specificity of this antibody for CYP2C19 over the closely related CYP2C9, purified IgG was back adsorbed against an acetone powder preparation (Maniatis et al., 1989) made from CYP2C9 in T. ni cell culture. The specificity of the anti-CYP2C19 IgG was evaluated by Western blot analysis (described below) using equipotent amounts of each recombinant CYP2C and the highest amounts of the remaining recombinant P450s used for the generation of the calibration curves for the quantification (P450s 1A1, 2C9, 2C19, 2D6, 2J2, 3A4, and 3A5) or for the detection (P450s 2A6, 2B6, and 2E1) of the various P450s in HIM.
Human Microsomes. Microsomes were prepared previously from mucosal scrapings obtained from the proximal portion (duodenum/proximal jejunum) of 31 human donor small intestines and from two human donor livers by standard ultracentrifugation methods (Paine et al., 1997; Madani et al., 1999). The use of these archived tissues for research purposes was approved by the University of North Carolina Institutional Review Board. For the 21 intestinal donors for which demographic and chronic drug and medical histories were available (Table 1), the majority was white and nine were men. In addition to potential chronic medications, organ donors often receive a number of medications before organ procurement that include various pressor, antiarrhythmic, and antimicrobial agents; insulin; and medications to treat brain injury, such as mannitol, dexamethasone, and phenytoin (Paine et al., 1997). The latter two agents are known P450 inducers.
Characteristics of the intestinal donors (n = 21) from whom relevant information was available
Western Blot Analysis. The total protein concentration of each microsomal preparation was remeasured by the method of Lowry et al. (1951), and the range of values agreed with those measured previously (Paine et al., 1997; Madani et al., 1999). Each intestinal preparation was diluted in sample buffer as described (Paine et al., 2005) to yield a final concentration of 5, 10, or 40 μg per 60 μl (Table 2). Likewise, the liver microsomes, used as positive controls for the detection of the various P450 enzymes, were diluted to final concentrations of 2 to 20 μg per 60 μl. To substantiate the average intestinal specific contents obtained for all of the P450s examined, except CYP3A5, aliquots (200 μl) from each of the 31 preparations were pooled; to substantiate average CYP3A5 content, aliquots from the 11 preparations with readily detectable CYP3A5 were pooled. The total protein concentration of each pooled sample was measured and diluted as described for the individual samples. Reference standards were prepared similarly using recombinant enzyme and matrix (i.e., a separate intestinal microsomal preparation that had undetectable protein when probed with each of the various anti-P450 antibodies). Diluted microsomes and reference standards were boiled for 3 min and loaded onto 0.1% SDS-9% polyacrylamide gels (14 × 16 cm), after which the proteins were electrophoretically separated and transferred overnight to polyvinylidene difluoride membranes as described previously (Paine et al., 2005).
Immunoblotting conditions for the detection of intestinal P450 isoforms
For detection of the various P450 enzymes, the blots were first placed in blocking buffer [5% nonfat dry milk in phosphate-buffered saline containing 0.3% Tween 20 (PBS-T)] at room temperature. After 1 h, the blots were rinsed in PBS-T, incubated with the anti-P450 primary antibody (Table 2), rinsed in PBS-T, incubated with the appropriate secondary antibody (Table 2), and rinsed again. The proteins of interest were visualized using enhanced chemiluminescence reagents and the Chemi-Doc imaging system (Bio-Rad). Integrated optical densities were obtained using the Bio-Rad software program Quantity One (version 4.1). Calibration curves were generated by plotting the integrated optical densities of the reference standards against the amount of recombinant enzyme loaded. The amount of each P450 enzyme/well was calculated relative to the calibration curve. Specific content was calculated by dividing the amount of enzyme/well by the amount of total protein loaded.
Statistical Analysis. Results are presented as means, ranges, and standard deviations (S.D.s). Comparisons of average P450 contents between the intestinal donors known to be men and women were made using the unpaired Student's t test. A p value < 0.05 was considered statistically significant.
Results
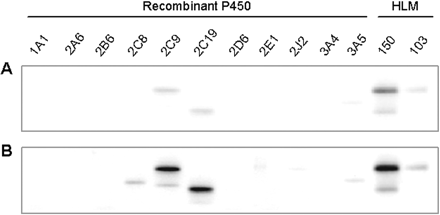
Specificity of the Anti-CYP2C19 Polyclonal Antibody. The anti-CYP2C19 IgG immunoreacted readily with both recombinant CYP2C9 and CYP2C19 (Fig. 1A). Because these two proteins were well separated from each other, as well as the corresponding bands in the two human liver microsomal preparations, the antibody was used to detect both CYP2C enzymes in the intestinal preparations. With prolonged exposure, the antibody cross-reacted with recombinant CYP2C8 and apparently CYP3A5 (Fig. 1B). The lack of detection of these two proteins in the human liver microsomal preparations was probably due to the relatively low mass of microsomal protein loaded (2 μg).
Variation in Proximal Small Intestinal P450 Enzymes. Because detailed medical and drug histories were not available for a large proportion (nearly one-third) of the donors, variations in the different P450 isoforms were evaluated without considerations of disease states and concomitant medications. As anticipated, CYP3A4 was detected readily in all donor intestines and exhibited large interindividual variation (17-fold) (Table 3). The polymorphic CYP3A5 was detected readily in 11 of the donors (35%), including those identified as African American (HI-2), Asian (HI-32), and Hispanic (HI-35). Variation in CYP3A5 content (5-fold) was lower compared with that for CYP3A4. CYP3A5 represented from 3 (HI-32) to 53% (HI-29) and averaged 27% of total CYP3A (CYP3A4+CYP3A5) content. Total CYP3A content for the 31 donors averaged 50 pmol/mg and ranged from 18 to 151 pmol/mg. Consistent with a recent report (Paine et al., 2005), a sex difference was not detected between the nine individuals known to be men and the 11 individuals known to be women for both CYP3A4 and CYP3A5 content (p = 0.65 and 0.84, respectively).
Specific P450 contents (picomoles per milligram microsomal protein) in human small intestinal microsomes (HIM)
Western blot showing the specificity of the anti-CYP2C19 polyclonal antibody after a typical exposure for the detection of CYP2C9 and CYP2C19 (A) or a prolonged exposure (B). Except for the recombinant CYP2Cs, which represent equimolar amounts (200 fmol), the amount of recombinant P450 represents the highest amount used for the generation of the calibration curves for quantification (P450s 1A1, 2D6, 2J2, 3A4, and 3A5) or detection (P450s 2A6, 2B6, and 2E1) in human intestinal microsomes: 100, 80, 100, 600, 400, 80, 150, and 40 fmol, respectively. Each human liver microsomal (HLM) preparation represents 2 μg of microsomal protein. HL-150 was known to contain CYP3A5.
Along with CYP3A4, P450s 2C9 and 2C19 were detected readily in all donor intestines and varied among individuals at least 5-fold (Table 3); with prolonged exposure, CYP2C8 was not detected in any of the donors (not shown). CYP2J2 was also detected readily in all donor intestines and varied at least 15-fold (Table 3). The polymorphic CYP2D6 was detected readily in 29 of the donors, including HIs 2, 32, and 35, and varied 7-fold. As with the CYP3A enzymes, no sex differences were detected for P450s 2C9, 2C19, and 2J2 (p ≥ 0.15). The predominantly extrahepatic CYP1A1 was detected readily in three of the donors (one known to be a white woman, 19A) and varied 2.1-fold (Table 3). The remaining P450 enzymes examined, P450s 1A2, 2A6, 2B6, and 2E1, were either not detected or detected only faintly, even after prolonged exposure.
In each of the 31 intestinal donors, CYP3A was the most abundant of the P450s examined. CYP2C9 was the second most abundant, followed generally by CYP2C19, and then by CYP2J2. In each of the 29 CYP2D6 expressors, CYP2D6 content was either similar to or lower than corresponding CYP2J2 content. On average, these five P450s followed the same rank order of abundance (Table 3).
To substantiate the average specific content, or lack of detection, of each P450 examined in the 31 intestinal donors (except CYP3A5), aliquots from each preparation were pooled and analyzed in triplicate. A second pooled preparation composed of the 11 CYP3A5 expressors was analyzed to substantiate average CYP3A5 content. Because CYP1A1 was detected in only three individuals, this enzyme was not detected in the pooled intestinal preparation (Fig. 2). The slower migrating bands detected in the representative human liver microsomes relative to the CYP1A1 reference standards were consistent with CYP1A2 having a slightly higher molecular mass compared with CYP1A1 (Paine et al., 1999). As with the individual intestines, P450s 2A6, 2B6, and 2E1 were not detected in the pooled preparation; in contrast, the representative liver microsomal preparations exhibited readily detectable bands that comigrated with the corresponding reference standards (Fig. 2). Likewise, the remaining enzymes were detected readily in both the pooled intestinal preparations and in the liver microsomes. The excellent agreement between the averages of the triplicate measurements of the various P450s in the pooled intestinal preparations and the average values determined from the individual preparations (Table 3) illustrated the robustness of the data.
Contributions of Individual P450 Enzymes to Total Proximal Small Intestinal P450 Content. Because of the limited quantities of the individual intestinal preparations, total P450 content could not be measured by difference spectra. Therefore, to obtain an estimate of total P450 content for each of the 31 intestinal donors, all immunoquantified P450s were summed. Total immunoquantified P450s averaged 61 pmol/mg and ranged from 22 to 180 pmol/mg. The excellent agreement between these values and those reported previously for total spectrally determined P450 content in proximal small intestinal microsomes [30–210 (Paine et al., 1997) and 60–180 pmol/mg (Zhang et al., 1999)] indicated that the P450s detected in the current work accounted for the majority of total P450 content. When expressed as a percentage of total immunoquantified P450s, CYP3A, CYP3A4, CYP2C9, CYP2C19, CYP2J2, and CYP2D6 ranged from 59 to 94, 33 to 87, 4 to 38, 0.5 to 7, 0.2 to 4, and 0.2 to 4%, respectively. The corresponding average contributions to the small intestinal P450 pie followed a similar trend (Fig. 3).
Western blots of pooled (n = 31, except where noted) human intestinal microsomal preparations (HIM) showing the presence or absence of various P450s. The amount of recombinant enzymes (standards) ranged from 100 to 12.5 (CYP1A1), 80 to 10 (CYP2A6), 150 to 18.8 (CYP2B6), 600 to 75 (CYP2C9), 200 to 25 (CYP2C19), 80 to 10 (CYP2D6), 40 to 5 (CYP2E1), 100 to 12.5 (CYP2J2), 600 to 75 (CYP3A4), and 400 to 50 (CYP3A5) fmol. The pooled HIM represent 40 (P450s 1A1, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and P2J2), 5 (CYP3A4), or 10 (CYP3A5) μg of microsomal protein. Each human liver microsomal (HLM) preparation represents 2 (CYP2E1), 5 (P450s 2A6, 2D6, 3A4, and 2J2), 10 (1A1, 2C9, 2C19, and 3A5), or 20 (CYP2B6) μg of microsomal protein. HL-150 was known to contain CYP3A5.
Measurement of total P450 content by difference spectra (Omura and Sato, 1964) was attempted with the pooled intestinal preparation containing microsomes from all 31 donors, but the suspension was too dilute to produce a reliable value. However, as with the individual preparations, the sum of total immunoquantified values (72 pmol/mg) was in excellent agreement with the average or median spectrally determined values reported previously for duodenal and jejunal microsomes (55–70 pmol/mg) (Watkins et al., 1987; Paine et al., 1997). When expressed as the percentage of contribution to the intestinal P450 pie, CYP3A4 was the most abundant (80%), followed by CYP2C9 (15%), CYP2C19 (2.9%), CYP2J2 (1.4%), and CYP2D6 (1%).
The average human proximal small intestinal and hepatic P450 pies. The percent contributions of individual P450 enzymes are based on total immunoquantified P450 content. The values for the liver pie were derived from Shimada et al. (1994).
Discussion
The oral route continues to serve as the most popular and convenient means of drug administration. Like the liver, the small intestine is replete with drug biotransformation enzymes, including the cytochromes P450. As a consequence, substantial presystemic metabolism can occur as the drug passes, sequentially, through the small intestine and liver. Although the relative contributions of individual P450s in the liver have been established for some time, less is known about the complement of enzymes in the proximal small intestine, the major site of xenobiotic absorption. Therefore, microsomes prepared from the duodenal/proximal jejunal portion of 31 human donor small intestines were analyzed by Western blot using selective P450 antibodies.
As anticipated, CYP3A represented the largest piece of the human intestinal P450 pie and exhibited large interindividual variation. CYP2C9 represented the second largest piece, followed by CYP2C19, CYP2J2, and CYP2D6. These enzymes also exhibited large interindividual variation, consistent with earlier reports involving smaller numbers of either microsomal or homogenate tissue specimens (Madani et al., 1999; Zhang et al., 1999; Matsumoto et al., 2002; Lapple et al., 2003). CYP1A1, which is expressed predominantly in extrahepatic tissues, was detected in only three individuals, including one known to be a white nonsmoking woman with hypertension. As reported previously (Paine et al., 1999), smoking status seemed not to influence intestinal CYP1A1 expression because the enzyme was not detected in any of the four donors known to be chronic smokers. Other CYP1A-inducible environmental chemicals, such as heterocyclic amines contained in the diet, including chargrilled meat (Fontana et al., 1999), may account for the CYP1A1 expression observed in these individuals. Dietary histories were not available for any of the current donors.
Because the pooled intestinal microsomal suspension was too dilute to obtain a reliable measure of total P450 content by difference spectra, the average specific contents determined for individual P450s were summed. Total immunoquantified P450 content (72 pmol/mg) was in excellent agreement with the average or median spectrally determined values reported previously for duodenal/jejunal microsomes (55–70 pmol/mg) (Watkins et al., 1987; Paine et al., 1997). As such, it is unlikely that other known (e.g., CYP2S1, CYP4F12) (Kaminsky and Zhang, 2003) or unknown P450s in the small intestine contribute substantially to total intestinal P450 content. To compare the P450 profile in the average small intestine with that in the average liver, the specific content of each enzyme/subfamily reported by Shimada et al. (1994) was expressed as the percentage of total immunoquantified P450 content. Because the seven hepatic enzymes examined accounted for 70% of total spectrally determined P450 content, the resulting values increased by approximately 40% (Fig. 3). Nevertheless, the contributions by the various P450s differed markedly between the two major organs involved in xenobiotic metabolism.
Although CYP3A was the largest piece of both pies, its contribution to the intestine was double its contribution to the liver and accounted for the majority (∼80%) of total P450 content. This observation, coupled with overlapping CYP3A content and associated catalytic activity between the two organs (Paine et al., 1997; Obach et al., 2001), substantiates the proximal small intestine as a major site for presystemic drug metabolism, as well as for drug-drug and drug-diet interactions.
CYP2C was the second largest piece of both P450 pies, with the percent contribution to the intestine being comparable with that to the liver (16 versus 25%). As evidenced from the current work and as reported by others (Lapple et al., 2003), CYP2C9 was the most abundant CYP2C enzyme in the intestine. CYP2C9 has also been reported as the most abundant CYP2C enzyme in the liver (Lasker et al., 1998). However, the average microsomal content in the intestine (∼8 pmol/mg) was an order of magnitude lower than that reported for the liver (89 pmol/mg) (Lasker et al., 1998). Average catalytic activity has also been reported to be lower (at least 4-fold) in enteric compared with liver microsomes (Prueksaritanont et al., 1996; Obach et al., 2001). These observations suggest that, in general, the intestine would have minimal contribution to the overall first-pass metabolism of CYP2C9 drug substrates. However, because of the wide range in both enteric CYP2C9 content and activity, enteric CYP2C9 could be important in some individuals for substrates with a low oral bioavailability, e.g., fluvastatin (Scripture and Pieper, 2001). The low expression of CYP2C9 in the intestine relative to the liver also does not preclude its potential importance to the first-pass metabolism of substrates ingested in trace amounts, e.g., pesticides (Hu et al., 2004; Usmani et al., 2004).
CYP2C19 was the third largest piece of the intestinal pie, representing only 2% of total immunoquantified P450s. In contrast, CYP1A2 was the third largest piece of the hepatic P450 pie. As with CYP2C9, the markedly lower average microsomal CYP2C19 content in the intestine (2 pmol/mg) relative to that in the liver (∼20 pmol/mg) (Lasker et al., 1998; Venkatakrishnan et al., 2000), along with much lower (at least 8-fold) catalytic activity (Obach et al., 2001), suggests that the intestine would also have minimal contribution to the overall first-pass metabolism of CYP2C19 drug substrates. Indeed, the list of CYP2C19 drug substrates with a low oral bioavailability is limited, suggesting that intestinal CYP2C19 generally does not contribute significantly to the first-pass metabolism of drugs. Again, this does not preclude the potential importance of enteric CYP2C19 to the first-pass metabolism of substrates ingested in trace amounts, e.g., pesticides and insect repellents (Tang et al., 2001; Usmani et al., 2002).
The relatively recently identified CYP2J2 (Zeldin et al., 1997) contributed <2% to the intestinal P450 pie. To our knowledge, the contribution of CYP2J2 to total hepatic P450 content is not known. CYP2J2 is particularly abundant in the heart, where it has been postulated to play a role in the cardioprotective effects of epoxyeicosatrienoic acids (EETs), the major CYP2J2-mediated metabolites of arachidonic acid (Scarborough et al., 1999). Human jejunal microsomes have also been demonstrated to produce EETs (Zeldin et al., 1997). In addition to the known effects of EETs on intestinal vascular tone, it has been speculated that these CYP2J2-mediated products have roles in the release of intestinal neuropeptides, control of intestinal motility, and/or modulation of intestinal fluid/electrolyte transport (Zeldin et al., 1997). Along with these potential physiologic roles, in vitro studies have suggested that enteric CYP2J2 may contribute to the first-pass metabolism of the nonsedating antihistamines astemizole (Hashizume et al., 2002) and ebastine (Matsumoto et al., 2002). Whether this occurs in vivo remains to be determined.
Intestinal CYP2D6 had minimal contribution to the intestinal P450 pie (<1%). Hepatic CYP2D6 also had minimal contribution to the hepatic P450 pie (2%). Despite these small contributions, several CYP2D6 substrates undergo extensive first-pass metabolism. The intestinal form, however, is unlikely to play a significant role. From a comprehensive characterization of human jejunal (n = 19) and hepatic (n = 31) microsomal preparations, median jejunal content was <8% of median hepatic content (0.9 versus 12.8 pmol/mg) (Madani et al., 1999). Median catalytic activity, as assessed by the intrinsic clearance of metoprolol oxidation, was also much lower in jejunal compared with hepatic microsomes (0.7 versus 19.7 μl/min/mg). Likewise, the predicted average in vivo intestinal extraction ratio for metoprolol was negligible compared with the predicted average hepatic extraction ratio (0.01 versus 0.48). Based on these observations, the authors concluded that unless a CYP2D6 substrate has a long residence time in the intestinal mucosa or undergoes futile cycling via an efflux transporter, intestinal CYP2D6 would be expected to contribute minimally to the overall first-pass metabolism of drugs. However, intestinal CYP2D6 may become clinically relevant if it mediates the formation of a cytotoxic metabolite that could lead to mucosal damage (Madani et al., 1999) (which could also be the case with intestinal CYP2Cs). In addition, as with the intestinal CYP2Cs, intestinal CYP2D6 could have an important role in the first-pass metabolism of substrates ingested in trace amounts.
Although only the proximal portion of the small intestine was examined in the current work, several P450s have also been detected in the more distal region, including CYP3A4, CYP3A5 (Kolars et al., 1994; Paine et al., 1997), CYP2C (de Waziers et al., 1990; Zhang et al., 1999), CYP2D6 (de Waziers et al., 1990; Madani et al., 1999), and CYP2J2 (Zeldin et al., 1997). Whereas CYP3A, CYP2C, and CYP2D6 content and associated catalytic activity tended to be highest in the proximal region and decline progressively toward the distal region, CYP2J2 seemed more uniform. The different pattern of CYP2J2 expression could be due in part to its lack of response to inducing agents, coupled with its documented expression in cell types in addition to the enterocytes, including the autonomic ganglion of nerves (Zeldin et al., 1997).
In summary, as in the human liver, large interindividual variation exists in the specific content of individual P450 enzymes in the human proximal small intestine (≥5-fold). However, the complement of drug-metabolizing P450s in the intestine is distinct from that in the liver. Of the 11 P450s examined, as expected, CYP3A accounted for the majority (∼80%) of total immunoquantified enteric P450 content and represented the largest piece of the intestinal P450 pie. CYP2C9 represented the second largest piece (∼15%), followed by CYP2C19 (2%), CYP2J2 (<2%), and CYP2D6 (<1%). Although these non-CYP3A enzymes are expressed and are catalytically active in the small intestine (along with CYP1A1 in some individuals), unlike CYP3A, their low specific contents relative to their hepatic counterparts question whether they contribute significantly to the first-pass metabolism of drugs. However, a role in the bioactivation or detoxification of other xenobiotics ingested in trace amounts cannot be excluded.
Footnotes
-
This work was supported in part by grants from Pfizer, Inc. and from the National Institutes of Health Grants GM32165 and GM38149.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.008672.
-
ABBREVIATIONS: P450, cytochrome P450; HIM, human intestinal microsome; PBS-T, phosphate-buffered saline containing 0.3% Tween 20; HI, human intestine; EET, epoxyeicosatrienoic acid.
- Received November 29, 2005.
- Accepted February 1, 2006.
- The American Society for Pharmacology and Experimental Therapeutics