Abstract
Linoleic acid has recently been shown to be glucuronidated in vitro by human liver and intestinal microsomes and recombinant UGT2B7. In the present study, the dietary fatty acids (FA), phytanic acid (PA), and docosahexaenoic acid (DHA) have been used as substrates for human UDP-glucuronosyltransferases (UGTs). Both compounds were effectively glucuronidated by human liver microsomes (HLM; 1.25 ± 0.36 and 1.12 ± 0.32 nmol/mg × min for PA and DHA, respectively) and UGT2B7 (0.71 and 0.53 nmol/mg × min). Kinetic analysis produced relatively low Km values for PA with both HLM and UGT2B7 (149 and 108 μM, respectively). TheKm for DHA glucuronidation by HLM (460 μM) was considerably higher than that for UGT2B7 (168 μM), suggesting the involvement in microsomes of other UGT isoforms in addition to UGT2B7. Glucuronidation of PA and DHA by gastrointestinal microsomes from 16 human subjects was determined. In general, both PA and DHA were glucuronidated by gastric and intestinal microsomes, and activity toward both substrates was lowest in the stomach, increased in the small intestine, and lower in the colon. However, there were large interindividual variations in UGT activity toward both substrates in all segments of the intestine, as has been seen with other substrates. Thus, PA and DHA are effective in vitro substrates for human liver, gastric and intestinal microsomes, and glucuronidation may play a role in modulating the availability of these FA as ligands for nuclear receptors.
FA1are important structural components of cell membranes, sources of energy, and precursors of eicosanoids. There is growing evidence supporting a role for FA in the modulation of cell-signaling pathways (Hwang and Rhee, 1999). Several recent studies have indicated that FA, such as PA and DHA (structures shown in Fig.1), activate the retinoid X (RXR) and peroxisome proliferator-activated (PPAR) nuclear receptors. PA is a branched-chain FA that is a constituent of dairy products, meat, and fish. Kitareewan et al. (1996) and Lemotte et al. (1996) simultaneously carried out studies that showed that PA and other phytol derivatives activate RXR. It has recently been shown that PA is also a naturally occurring ligand for PPARα (Zomer et al., 2000). DHA is a constituent of the human diet, particularly fatty fish, and plays physiological roles in brain maturation and development and retina development (de Urquiza et al., 2000; Kastner et al., 1994). Studies have demonstrated that DHA is a highly specific ligand for RXRα in mouse brain (de Urquiza et al., 2000), and DHA has been reported as a ligand for PPARα (Keller et al., 1993).
Structures of phytanic and docosahexaenoic acid.
Although glucuronides of medium-chain and oxidized long-chain FA have been identified as being excreted in human urine (Duran et al., 1985;Kuhara et al., 1986; Costa et al., 1996; Street et al., 1996; Sacerdoti et al., 1997), only recently has glucuronidation of FA been demonstrated in vitro with human liver and intestine (Jude et al., 2000, 2001a,b). In the current studies, we investigated the glucuronidation of PA and DHA by human liver and intestinal microsomes and recombinant UGT2B7, which has been shown to glucuronidate linoleic acid (LA; cis-9,12-octadecadienoic acid) and its oxidized derivatives (Jude et al., 2001a,b). The results showed that both PA and DHA were effectively glucuronidated in vitro by human liver and intestinal microsomes and UGT2B7.
Materials and Methods
PA, DHA, UDP-glucuronic acid (UDPGA), saccharolactone, and HEPES were obtained from Sigma Chemical Co. (St. Louis, MO). [Glucuronyl-14C]UDPGA ([14C]UDPGA) was from PerkinElmer Life Sciences (Boston, MA). Solvents for thin layer chromatography were all high-pressure liquid chromatography grade (Fisher Scientific, Houston, TX). All other reagents were of the highest purity available.
Human Liver Microsomes and Human Recombinant UGT2B7.
Human liver and gastrointestinal microsomes were prepared as previously described (Jude et al., 2001b) from tissue from organ donors obtained by transplant surgeons at University Hospital (Little Rock, AR) according to a protocol approved by the Human Research Advisory Committee of the University of Arkansas for Medical Sciences. HK293 cells expressing UGT2B7, prepared as previously described (Coffman et al., 1997), were a gift from Dr. T. Tephly, Department of Pharmacology (University of Iowa, Des Moines, IA). A membrane fraction enriched in UGT2B7 was prepared as described by Battaglia et al. (1994), and aliquots were stored at −80°C until used. Dr. Tephly also supplied membrane fractions enriched in human UGT1A8 and UGT1A10.
Enzyme Assays.
UGT activities were determined as described in detail previously (Radominska-Pyrek et al., 1986, 1987) using [14C]UDPGA as the sugar donor, 100 μM PA and DHA as substrates, and 50 μg of microsomal protein, with incubation at 37°C for 10 to 30 min. PA and DHA were prepared as micelles with Brij 58, which served to both solubilize the substrates and activate UGTs. Kinetic analysis was carried out at a constant UDPGA concentration (4 mM), with substrate concentrations from 25 to 750 μM and an incubation time of 10 min. Kinetic parameters were determined using EnzymeKinetics software (Trinity Software, Compton, NH).
For β-glucuronidase hydrolysis, after the standard assay procedure, duplicate reactions were stopped with 1 ml of 0.1 M glycine-trichloroacetic acid, pH 2.8, and samples were applied to BondElut C18 cartridges (Varian, Palo Alto, CA) primed as recommended by the manufacturer. The cartridges were washed with glycine-trichloroacetic acid (5 ml) and water (5 ml) and eluted with methanol (3 ml); the methanol was evaporated. For each set of duplicate samples, one was dissolved in 30 mM sodium phosphate buffer, pH 7.4, containing 50 units of Escherichia coliβ-glucuronidase, and the other sample was dissolved in buffer alone (60 μl). Both samples were incubated for 4 h at 37°C after which reactions were stopped with ethanol. The samples were then handled as for a standard assay.
Results and Discussion
The results of assays of enzymatic activity toward PA and DHA with HLM and UGT2B7 demonstrated that both PA and DHA were good substrates for microsomal UGTs and UGT2B7. The UGT activities toward each substrate were similar for each protein source (PA: 1250 ± 357 and 1119 ± 320 pmol/mg of protein × min for HLM2 and UGT2B7, respectively; DHA: 710 ± 122 and 533 ± 87 pmol/mg of protein × min for HLM2 and UGT2B7, respectively) and are within the same range as those found in our laboratory for other FA (Jude et al., 2000, 2001a,b). As with other FA, the activity toward both PA and DHA was significantly higher with HLM than with UGT2B7, suggesting that UGT isoforms other than UGT2B7 are involved in the glucuronidation of these FA. Human recombinant UGT1A8 and UGT1A10, intestine-specific isoforms, had no measurable activity toward PA or DHA.
To confirm that the polar metabolites were indeed glucuronides, PA and DHA were incubated with HLM2 under standard assay conditions, and the products were partially purified on C18cartridges, followed by incubation at 37°C in absence or presence of β-glucuronidase. There was significant activity in the absence of β-glucuronidase (1410 and 994 pmol/mg × min for PA and DHA, respectively). However, following β-glucuronidase hydrolysis, there was no detectable activity with either substrate. Since the only functional group in these compounds available for conjugation is the carboxyl group, these metabolites are carboxyl-linked glucuronides, which have also been identified for LA and 13-hydroxyoctadecadienoic acid (Jude et al., 2001a).
Kinetic analysis (Table 1) showed that the apparent Km values for glucuronidation of PA by HLM2 and UGT2B7 were similar, but theVmax for HLM2 was more than twice that of UGT2B7. These values are very similar to those found previously for LA (Jude et al., 2001a). With DHA, theKm for UGT2B7 was more than 2 times lower than that for HLM2, reinforcing the inference from the enzymatic assays that more than one UGT isoform is involved in the microsomal glucuronidation of DHA. The DHA Vmaxwith HLM2 was 4 times higher that than found with UGT2B7. Despite the variations in Km andVmax, the efficiencies of the reactions (Vmax/Km) were similar, varying over only a 2-fold range.
Apparent kinetic parameters for the glucuronidation of PA and DHA by human liver microsomes (HLM2) and membrane fractions of HK293 cells expressing UGT2B7
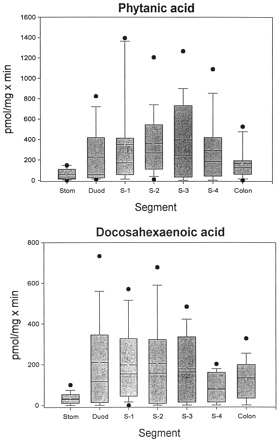
We have shown previously that LA and its hydroxylated derivatives, LA-9,10-diol and LA-12,13-diol, are glucuronidated by human intestinal microsomes (Jude et al., 2001b). Since both PA and DHA are dietary FA and are therefore exposed to the intestinal mucosa, we measured the glucuronidation of these two compounds by microsomes from the stomach, small intestine (in 4 segments), and colon of 16 human subjects (5 females and 11 males; not all segments were available for all subjects). The results of these assays are presented in Fig.2. Several things are apparent from these data. First, there were very large interindividual variations in activity toward both substrates, as has been demonstrated previously with LA and the LA diols (Jude et al., 2001b), steroid hormones, retinoic acid, and 4-nitrophenol (Radominska-Pandya et al., 1998;Czernik et al., 2000). Second, although the mean glucuronidation levels toward the two substrates were not, in general, hugely different, the maximum activities were almost twice as high for PA as for DHA. The lowest activity for both substrates was found in stomach. For PA, glucuronidation generally increased somewhat from duodenum through S-3 and then decreased slightly in S-4, with an additional decrease in colon. Glucuronidation of DHA was fairly constant from duodenum through S-3 but decreased by about half in S-4, with another slight increase in colon. Additionally, glucuronidation of both substrates by gastrointestinal microsomes from a majority of the subjects was lower than comparable values measured with liver microsomes.
Glucuronidation of phytanic and docosahexaenoic acid by human gastrointestinal microsomes.
Microsomes prepared from stomach (Stom), duodenum (Duod), four segments of the remaining small intestine (S-1 to S-4), and colon from human subjects were assayed for glucuronidation activity toward PA and DHA, as described under Materials and Methods. In the box plot shown, the boxes are limited by the 25th and 75th percentiles. The solid line in each box gives the median, and the broken line shows the mean. The brackets cover the 10th to 90th percentiles, and the filled circles shown are outliers.
Although intestinal glucuronidation levels for both substrates were much lower than in liver microsomes, when the size of the intestine is taken into account, these data indicate that there is likely to be considerable first-pass metabolism of both PA and DHA in human intestine. Recent work from several laboratories has shown that drug-metabolizing enzymes are actively involved in protecting the organism from absorption of harmful chemicals, both endogenous and exogenous (Hall et al., 1999; Lin et al., 1999; Racissi et al., 1999;Schuetz and Schinkel, 1999). The cytochromes P450 and UGTs work in concert to biotransform toxic compounds into forms which are substrates for the ATP-dependent transporters, such as P-glycoprotein, multispecific organic anion transporter, and multidrug resistance 1, 2, and 3 (Schinkel, 1998; Smit et al., 1998), and thus can be excreted out of the intestinal mucosa. This function would be especially important in relation to dietary FA, such as PA and DHA. The active glucuronidation of FA by intestinal UGTs may be involved in preventing accumulation of excess FA by contributing to the excretion of glucuronidated FA from the intestinal mucosa back into the lumen. Additionally, it has been hypothesized that drug-metabolizing enzymes, including UGTs, play a role in controlling concentrations of ligands for nuclear receptors (Nebert, 1991). Since PA and DHA are effective ligands for RXRα and PPARα within the cell, glucuronidation may function to limit the availability of PA and DHA as ligands for nuclear receptors.
Footnotes
-
This research was supported in part by National Institutes of Health Grant DK56226 to A.R.-P.
- Abbreviations used are::
- FA
- fatty acids
- PA
- phytanic acid (3,7,11,15-tetramethylhexadecanoic acid)
- DHA
- cis-4,7,10,13,16,19-docosahexaenoic acid
- RXR
- retinoid X receptor
- PPAR
- peroxisome proliferator-activated receptor
- LA
- linoleic acid (cis-9,12-octadecadienoic acid)
- UDPGA
- UDP-glucuronic acid
- UGT
- UDP-glucuronosyltransferase
- HLM
- human liver microsomes
- Received October 19, 2001.
- Accepted January 24, 2002.
- The American Society for Pharmacology and Experimental Therapeutics