Abstract
The objectives of the study were to characterize the selectivity of dantrolene to breast cancer resistance protein (Bcrp) and to evaluate whether cerebrospinal fluid (CSF) can be used as a surrogate to assess brain exposures of BCRP and P-glycoprotein (Pgp) substrates. The impact of Bcrp and Pgp on dantrolene exposures in brain and CSF was examined in Bcrp and Mdr1a/1b knockout mice and was further investigated in wild-type mice in the presence of the Bcrp inhibitor (3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester (Ko143), the Pgp inhibitor 6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-l-valine-cyclosporine A (PSC833), and the dual inhibitor N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918). The effect of Bcrp and Pgp on digoxin exposures in brain and CSF was investigated in wild-type mice in the presence of the inhibitors. In vivo studies showed dantrolene exposures in brain and CSF, but not the blood, increased in Bcrp(−/−) and Mdr1a/1b(−/−)/Bcrp(−/−) mice, or in the presence of the Bcrp inhibitors Ko143 or GF120918. Inhibition of Pgp by GF120918 and PSC833 significantly increased digoxin exposures in brain, CSF, and blood to a lesser extent. Results from the present study demonstrated that inhibition of Bcrp and Pgp increased not only the exposures of dantrolene and digoxin in brain, but also the exposures in CSF. In addition, the change of exposures in CSF reflected the changes in brain. The present study strongly suggests that the dantrolene and digoxin exposures in CSF are primarily determined by the rapid transport from brain to CSF, and inhibition of Bcrp and Pgp exhibits little impact on using CSF as surrogates to assess brain exposures of Bcrp and Pgp substrates.
Introduction
The expression of P-glycoprotein (Pgp) on the luminal membrane of brain endothelial microvessel cells has been well characterized (Demeule et al., 2002; Ueno, 2009; Reichel et al., 2011). Various studies have demonstrated that the penetration of a vast variety of drugs across the blood-brain barrier (BBB) is determined by Pgp (Schinkel et al., 1997; Chen et al., 2003; Ose et al., 2008). Similar to Pgp, the expression of Bcrp on the luminal membrane of brain endothelial cells has been demonstrated (Daood et al., 2008; Roberts et al., 2008). It has been shown that Pgp and breast cancer resistance protein (Bcrp) work in concert in restricting drug penetration to the brain (Polli et al., 2009; Kodaira et al., 2010). However, for cosubstrates of both Bcrp and Pgp, the magnitude of Bcrp in restricting drug penetration to the brain is generally not as profound as that of Pgp, likely because of the higher expression of Pgp at the BBB (Breedveld et al., 2005; Bihorel et al., 2007; de Vries et al., 2007; Giri et al., 2008; Chen et al., 2009). The identification and characterization of a Bcrp selective substrate, therefore, become necessary when investigating the function of Bcrp in regulating drug transport to the brain, as well as evaluating Bcrp-mediated drug-drug interactions.
The brain is protected by two barriers, the BBB and the blood cerebrospinal fluid (CSF) barrier (BCSFB). To deliver drugs to the brain, systemically administrated drugs have to pass the BBB and/or the BCSFB. Previous studies have shown that CSF can be used as surrogates to assess free drug concentrations in brain (Liu et al., 2006, 2009; Lin, 2008). The expression of Pgp, but not Bcrp, on the luminal membrane of choroid plexus epithelial cells has been reported (Rao et al., 1999; Daood et al., 2008). Results from other studies indicated that both Bcrp and Pgp (Zhuang et al., 2006) or neither Bcrp nor Pgp (Roberts et al., 2008) is expressed in choroid plexus cells. Cellular localization of ATP-binding cassette transporters in brain endothelial cell monolayer and choroid plexus epithelial cell monolayer is crucial in determining drug transport to the brain and CSF. The localization of Pgp at the luminal side of the choroid plexus suggests it would facilitate but not restrict drug transport to CSF. It is therefore anticipated that Pgp impairment will decrease drug penetration across BCSFB. Because of the opposite effects of Pgp inhibition on drug transport to the brain and CSF, CSF may not accurately represent the exposures in brain if the compound in CSF is mainly determined by the transport from blood across the BCSFB. Whereas the function of Pgp and Bcrp in regulating drug transport across BBB has been well characterized (Demeule et al., 2002; Daood et al., 2008; Roberts et al., 2008; Ueno, 2009; Reichel et al., 2011), it is still not clear whether Bcrp and Pgp could affect drug transport across the BCSFB. Whether CSF can be used as a surrogate to assess brain exposures of Bcrp and Pgp substrates, therefore, needs to be further examined. It was reported that inhibition of Pgp by gefitinib increased topotecan exposure in brain but decreased the exposure in the CSF, although the impact of Pgp inhibition on CSF exposures was not significant (Zhuang et al., 2006). Studies using knockout animals found the CSF exposure of a Pgp substrate loperamide was either not changed in Mdr1(−/−) dogs (Mealey et al., 2008) or increased in Mdr1a/1b(−/−) mice (Doran et al., 2005). The discrepancy among the studies could be due to the nonspecific Pgp substrates and inhibitors used in the studies, so the contributions from other transporters cannot be ruled out. The discrepancy could also be due to differences in Pgp expression levels on choroid plexus between dog and mouse or between mice at different ages because at least it is known that the expression level of Pgp in human choroid plexus changed during the lifespan (Daood et al., 2008). In the present study, dantrolene as a relatively specific substrate of BCRP was characterized, and digoxin was selected as the prototypical substrate of Pgp. The effect of Bcrp and Pgp on dantrolene and digoxin transport to the brain and CSF was investigated by inhibiting the transporters with specific inhibitors or by using Bcrp and Pgp gene knockout mice. The objectives of this study are to gain a better understanding of the function of Bcrp and Pgp in regulating drug transport to the brain and CSF and, more importantly, to evaluate whether CSF can be used as surrogates to assess brain exposures of Bcrp and Pgp substrates.
Materials and Methods
Chemicals.
Dantrolene, digoxin, estrone-3-sulfate, and (E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid (MK571) were purchased from Sigma-Aldrich (St. Louis, MO). 6-[(2S,4R,6E)-4-Methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-l-valine-cyclosporine A (PSC833) was purchased from XenoTech, LLC (Lenexa, KS). [3H]Digoxin (35.4 Ci/mmol) and [3H]estrone-3-sulfate (54.3 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). (3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester (Ko143) and N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) were synthesized as customer products. All other chemicals and reagents were the highest grades that are commercially available.
Bidirectional Transport Assay Using Caco-2 System.
Caco-2 cells were purchased from the American Type Culture Collection (Manassas, VA). Caco-2 cells were grown with media containing high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% nonessential amino acids. Caco-2 cells from passage 25 to 50 were seeded on a 24-well Transwell plate (BD Biosciences Pharmingen, San Diego, CA). Caco-2 cells were washed with transport buffer containing Hanks' balanced salt solution, 25 mM HEPES, pH 7.4, and substrate was added to the apical chamber to measure transport to the basolateral chamber (A-B transport) or to the basolateral chamber to measure transport to the apical chamber (B-A transport) and was incubated for 2 h at 37°C with gentle shaking. For inhibition studies, Caco-2 cells were washed and preincubated for 30 min at 37°C with inhibitors present on both apical and basolateral chambers in transport buffer containing Hanks' balanced salt solution, 25 mM HEPES, pH 7.4, and 1% dimethyl sulfoxide. For the remainder of the experiment, inhibitors were present in both apical and basolateral chambers.
Animals.
Wild-type FVB mice were purchased from Charles River Laboratories (Wilmington, MA). Bcrp(−/−), Mdr1a/1b(−/−), and Mdr1a/1b(−/−)/Bcrp(−/−) FVB mice were ordered from Taconic Farms (Germantown, NY).
Drug Administration and Sample Collection.
Dantrolene and digoxin were prepared in 10% Captisol (Cydex Pharmaceutical Inc., La Jolla, CA) to yield solutions of 0.4 and 0.2 mg/ml, respectively. Dantrolene (2 mg/kg) and digoxin (1 mg/kg) were administrated via tail vein injection. For dantrolene, blood, brain, and CSF samples were collected at 5, 30 min, 1, 2, and 3 h (n = 3). For digoxin, blood, brain, and CSF samples were collected at 5, 30 min, 2, and 6 h (n = 3). Animals were euthanized by CO2 inhalation, and CSF was collected via cisterna magna puncture, using an insulin syringe. Blood was collected via cardiac puncture. The animals were then perfused with 5 ml of 0.9% sterile saline before brains were removed from the skull, transferred to Covaris tissue bags (Covaris, Woburn, MA), and weighed. All samples were stored at −80°C. To examine the effect of inhibitors on brain and CSF distribution, mice were preinjected with GF120918 (10 mg/kg), Ko143 (10 mg/kg), or PSC833 (10 mg/kg) solutions 5 min before dantrolene or digoxin administration. GF120918, Ko143, and PSC833 were dissolved in EtOH/PG200/5% glucose (2:6:2) at a concentration of 1 mg/ml.
Dantrolene and Digoxin Concentration Determination.
Frozen tissue samples were crushed with the CryoPrep pulverizer (Covaris). The frozen powder was transferred to glass vials where water was added (150 μl/100 mg tissue) and then was immediately homogenized for 30 s using the Covaris E200 focused acoustic device. An equal volume of acetonitrile was added, and samples were homogenized for an additional 30 s. Homogenates were centrifuged, and 60 μl of supernatant plus 5 μl of 8-cyclopently-1,3-dipropylxanthine (CPDPX; internal standard at 50 ng/ml) were added to 300 μl of acetonitrile/water/formic acid (20:80:0.1).
Whole-blood and CSF samples were processed in the following manner. Fifty microliters of whole-blood samples were first mixed with 30 μl of 0.1% formic acid and 5 μl internal standard (CPDPX at 50 ng/ml), and 5 μl of the same internal standard was added to 10 μl of CSF samples. Both whole-blood and CSF samples (containing internal standard) were precipitated with 150 μl acetonitrile/methanol (1:1). After centrifugation, the entire supernatant was removed and added to 150 μl of 0.1% formic acid. Samples were analyzed by liquid chromatography/tandem mass spectrometry (LC/MS/MS) using multiple reaction monitoring. A high-performance liquid chromatography system, fitted with a Kinetex C18 2.6 μm (3.0 × 50 mm) column (Phenomenex, Torrance, CA), was operated in gradient mode from 90% mobile phase A (water/formic acid, 100:0.1, v/v) to 95% mobile phase B (acetonitrile/formic acid, 100:0.1, v/v) over 2 min. The samples, along with an internal standard, were analyzed on a triple quadrupole mass spectrometer (Sciex API 5500; Applied Biosystems/MDS Sciex, Foster City, CA) that was equipped with a turbo ion spray probe and operated in negative ion mode. The data were collected and processed using Analyst version 1.5.1 (Applied Biosystems/MDS Sciex). All calculations were based on peak area ratios between dantrolene/digoxin, and the internal standard and sample concentrations were determined from the linear range of the standard curve.
Statistics and Data Analysis.
Experiments were repeated several times, and data from one representative experiment were presented. Plasma, brain, and CSF concentrations were presented as mean ± S.D. (n = 3). Blood, brain, and CSF pharmacokinetic parameters were calculated with noncompartment analysis using WinNonlin 6.1 (Pharsight, Mountain View, CA). The area under the concentration curve (AUC) from 0 to last time point was calculated using the linear trapezoidal method. Bidirectional transport data from the Caco-2 system were presented as mean ± S.E. (n = 2). Statistical analysis of A-B and B-A transport was performed by comparing the results in the presence of inhibitors with those from control in the same experiments using a two-tailed, two-sample equal variance t test. Results with a probability of P < 0.05 were considered significantly different.
Results
Bidirectional Transport of Dantrolene across Caco-2 Monolayers.
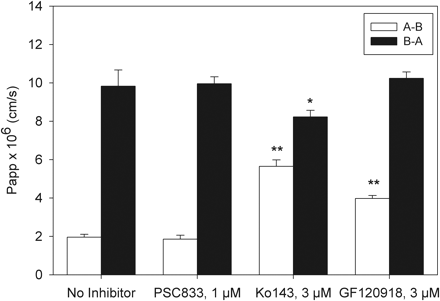
The selectivity of dantrolene as a Bcrp substrate was examined in vitro. The A-B and B-A transport of dantrolene across Caco-2 monolayers was determined in the absence and presence of BCRP-specific inhibitor Ko143, Pgp-specific inhibitor PSC833, and the Pgp/BCRP dual inhibitor GF120918 (Fig. 1). In the absence of the inhibitors, dantrolene exhibited low to moderate permeability under the experimental conditions. The A-B and B-A permeability was 2.0 × 10−6 cm/s and 9.8 × 10−6 cm/s, respectively, and the B-A/A-B ratio was approximately 5, which suggested dantrolene is subjected to an efflux transport. Expression of BCRP and Pgp in the Caco-2 system has been confirmed (data not shown). To examine whether the transport of dantrolene was mediated by BCRP and/or Pgp, the effect of BCRP- and Pgp-specific inhibitors on the transport of dantrolene was examined. In the presence of the BCRP-specific inhibitor Ko143, the A-B transport increased, whereas B-A transport decreased, resulting in an overall decrease in the B-A/A-B ratio. In the presence of the Pgp/Bcrp dual inhibitor GF120918, dantrolene A-B transport increased significantly, whereas the B-A transport did not change. In contrast, the Pgp-specific inhibitor PSC833 had no significant effect on the A-B and B-A transports. In vitro results indicated that dantrolene is a substrate of BCRP, but not Pgp.
Bidirectional transport of dantrolene across Caco-2 monolayers in the absence and presence of inhibitors. Dantrolene concentration was 5 μM. Open bars, A-B transport; closed bars, B-A transport. Data are presented as means ± S.E. *, P < 0.05; **, P < 0.001.
Dantrolene Inhibition on the Bidirectional Transport of Estrone-3-Sulfate across Caco-2 Monolayers.
To further confirm the selectivity of dantrolene to BCRP, the inhibitory effect of dantrolene on BCRP- and Pgp-mediated bidirectional transport across Caco-2 monolayers was investigated. Estrone-3-sulfate and digoxin were selected as in vitro probe substrates of BCRP and Pgp, respectively. Pgp-mediated transport of digoxin was demonstrated by the inhibition with Pgp-specific inhibitor PSC833 or the Pgp/BCRP dual inhibitor GF120918, but not the BCRP-specific inhibitor Ko143 (Fig. 2A). Likewise, BCRP-mediated transport of estrone-3-sulfate across Caco-2 monolayers was demonstrated by the inhibition with the BCRP-specific inhibitor Ko143 or the Pgp/BCRP dual inhibitor GF120918, but not the Pgp-specific inhibitor PSC833 (Fig. 2 B). As shown in Fig. 2, dantrolene substantially inhibited the transport of estrone-3-sulfate and reduced the B-A/A-B ratio close to unity. In contrast, dantrolene exhibited much less inhibition on the transport of digoxin. The results indicated dantrolene is an inhibitor of BCRP, but not Pgp.
Bidirectional transport of [3H]digoxin (A) and [3H]estrone-3-sulfate (B) across Caco-2 monolayers in the absence and presence of inhibitors. Digoxin and estrone-3-sulfate concentrations were 5 and 1 μM, respectively. Open bars, A-B; closed bars, B-A transport. Data are presented as means ± S.E. *, P < 0.05; **, P < 0.001.
Blood, Brain, and CSF Distribution of Dantrolene in Mdr1a/1b and Bcrp Knockout Mice.
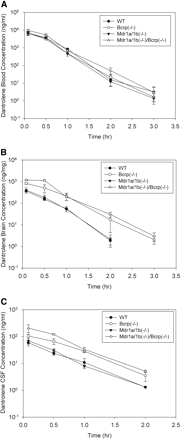
The effect of Bcrp and Pgp on dantrolene transport to the brain and CSF was further investigated in Bcrp and Mdr1a/1b gene knockout mice. Bcrp(−/−), Mdr1a/1b(−/−), and Mdr1a/1b(−/−)/Bcrp(−/−) FVB mice were dosed intravenously with dantrolene (2 mg/kg) as described under Materials and Methods. Dantrolene concentrations in blood, brain, and CSF were determined by LC/MS/MS (Fig. 3). Dantrolene AUC changes in knockout mice are summarized in Table 1. Compared with the wild-type mice, dantrolene blood concentrations changed by less than 50% in Bcrp(−/−), Mdr1a/1b(−/−), and Mdr1a/1b(−/−)/Bcrp(−/−) mice (Fig. 3A). Dantrolene blood AUC decreased slightly in Bcrp(−/−) and Mdr1a/1b(−/−) mice and increased by 42% in Mdr1a/1b(−/−)/Bcrp(−/−) mice (Table 1). Mdr1a/1b gene knockout had little impact on dantrolene brain and CSF exposures. Dantrolene AUC increased by less than 20% in Mdr1a/1b(−/−) mice (Fig. 3, B and C; Table 1). In contrast, dantrolene brain and CSF AUC increased 2- to 4-fold in Bcrp(−/−) mice and 3- to 6-fold in Mdr1a/1b(−/−)/Bcrp(−/−) mice, respectively (Fig. 3, B and C; Table 1). Dantrolene brain and CSF AUC also increased 2- to 4-fold in Bcrp(−/−) mice and 3- to 5-fold in Mdr1a/1b(−/−)/Bcrp(−/−) mice, respectively (Table 1). In both Bcrp(−/−) and Mdr1a/1b(−/−)/Bcrp(−/−) mice, dantrolene exposures in CSF increased to an extent similar to those in brain (Table 1). In addition to the exposure changes in blood, brain, and CSF, the effect of Bcrp and Mdr1a/1b knockout on dantrolene brain/blood AUC and CSF/blood AUC ratios was analyzed. Dantrolene brain/blood AUC and CSF/blood AUC ratios significantly increased in Bcrp(−/−) and Mdr1a/1b(−/−)/Bcrp(−/−) mice but not in Mdr1a/1b(−/−) mice (Fig. 4). Compared with the wild-type mice, the brain/blood and CSF/blood AUC ratios increased >3-fold and >2-fold, respectively, in Bcrp(−/−) and Mdr1a/1b(−/−)/Bcrp(−/−) mice, but only slightly increased in Mdr1a/1b(−/−) mice (Table 1). Finally, the impact of gene knockout on dantrolene CSF/brain AUC ratio was evaluated. In contrast to the marked increase in brain/blood and CSF/blood AUC ratios, dantrolene CSF/brain AUC ratio was not affected overall. The CSF/brain AUC ratio in Bcrp(−/−) and Mdr1a/1b(−/−)/Bcrp(−/−) mice decreased only slightly (25%), and the ratio remained the same in Mdr1a/1b(−/−) mice (Table 1).
Dantrolene concentration time profiles of blood (A), brain (B), and CSF (C) in wild-type FVB mice, Bcrp(−/−) knockout mice, Mdr1a/1b(−/−) knockout mice, and Mdr1a/1b(−/−)/Bcrp(−/−) knockout mice after intravenous dosing of dantrolene (2 mg/kg). Closed circles, concentrations in wild-type FVB mice; open circles, concentrations in Bcrp(−/−) knockout mice; closed triangles, concentrations in Mdr1a/1b(−/−) knockout mice; open triangles, concentrations in Mdr1a/1b(−/−)/Bcrp(−/−) knockout mice. Data are presented as mean ± S.D.
Dantrolene AUC changes in wild-type and knockout mice
Dantrolene blood, brain, and CSF AUC in wild-type and Mdr1a/1b and Bcrp knockout mice were determined, and AUC ratios were calculated.
Effects of Bcrp and Pgp on dantrolene distribution in blood, brain, and CSF. Dantrolene AUC in plasma, brain, and CSF was determined from wild-type FVB mice, Bcrp(−/−) knockout mice, Mdr1a/1b(−/−) knockout mice, and Mdr1a/1b(−/−)/Bcrp(−/−) knockout mice. Brain/blood, CSF/blood, and CSF/brain AUC ratios in wild-type mice and knockout mice were calculated. Open bars, wild-type FVB mice; dotted bars, Bcrp(−/−) knockout mice; diagonal bars, Mdr1a/1b(−/−) knockout mice; black bars, Mdr1a/1b(−/−)/Bcrp(−/−) knockout mice.
Blood, Brain, and CSF Distribution of Dantrolene in Wild-Type FVB Mice in the Presence of Inhibitors.
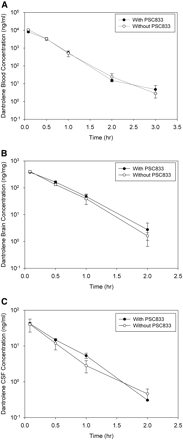
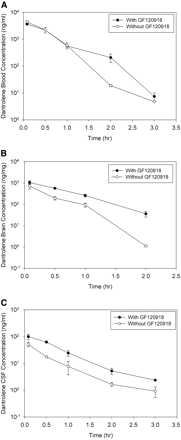
The effect of Bcrp and Pgp on dantrolene transport to the brain and CSF was further investigated by blocking the transporters with chemical inhibitors. Wild-type FVB mice were predosed intravenously with vehicle, Ko143 (10 mg/kg), GF120918 (10 mg/kg), or PSC833 (10 mg/kg) and then were dosed intravenously with dantrolene (2 mg/kg) as described under Materials and Methods. Dantrolene concentrations in blood, brain, and CSF were determined by LC/MS/MS (Figs. 5⇓–7). Dantrolene AUC changes in the presence of the inhibitors are summarized in Table 2. Compared with the control group, dantrolene blood concentrations and AUC did not change significantly in the presence of Bcrp and Pgp inhibitors GF120918, Ko143, and PSC833. Dantrolene blood AUC did not change in the presence of GF120918 and Ko143 and decreased slightly (16%) in the presence of PSC833 (Table 2). Dantrolene brain and CSF concentrations and AUC did not change in the presence of the Pgp inhibitor PSC833 (Table 2; Fig. 5) but increased in the presence of the Bcrp inhibitor Ko143 or the Pgp/Bcrp dual inhibitor GF120918 (Table 2; Figs. 6 and 7). Dantrolene concentrations in brain and CSF increased 2- to 4-fold, and dantrolene AUC in brain and CSF increased 2- to 3-fold in the presence of GF120918 and Ko143 (Table 2). Compared with the control mice, dantrolene brain/blood and CSF/blood AUC ratios increased 2- to 3-fold in the presence of the Bcrp inhibitor Ko143 or the Pgp/Bcrp dual inhibitor GF120918 but increased by less than 40% in the presence of the Pgp inhibitor PSC833 (Table 2). Because of the similar extent of increased exposures in brain and CSF, dantrolene CSF/brain AUC ratio did not change in the presence of the inhibitors. The CSF/brain AUC ratio increased by less than 30% in the presence of Ko143, PSC833, and GF120918 (Table 2). Results from the study indicated whereas the Bcrp inhibitor Ko143 and the Pgp/Bcrp dual inhibitor GF120918 increased the brain/blood and CSF/blood AUC ratios, the inhibitors had no significant impact on the CSF/brain AUC ratio of dantrolene.
Dantrolene concentration time profiles of blood (A), brain (B), and CSF (C) in wild-type FVB mice after intravenous dosing of dantrolene (2 mg/kg). Open circles, predosed intravenously with vehicle; closed circles, predosed intravenously with Ko143 (10 mg/kg). Data are presented as mean ± S.D.
Dantrolene concentration time profiles of blood (A), brain (B), and CSF (C) in wild-type FVB mice after intravenous dosing of dantrolene (2 mg/kg). Open circles, predosed intravenously with vehicle; closed circles, predosed intravenously with PSC833 (10 mg/kg). Data are presented as mean ± S.D.
Dantrolene concentration time profiles of blood (A), brain (B), and CSF (C) in wild-type FVB mice after intravenous dosing of dantrolene (2 mg/kg). Open circles, predosed intravenously with vehicle; closed circles, predosed intravenously with GF120918 (10 mg/kg). Data are presented as mean ± S.D.
Dantrolene AUC changes in wild-type mice predosed intravenously with vehicles or inhibitors
Dantrolene blood, brain, and CSF AUC in wild-type mice in the absence and presence of the inhibitors were determined, and AUC ratios were calculated.
Pharmacokinetics and Distribution of Digoxin in Wild-Type Mice.
Digoxin as a selective substrate of Pgp in vivo has been well established. The present study was focused on the effect of Bcrp and Pgp on digoxin transport to brain and CSF, particularly to investigate the relative change in brain and CSF exposures when Bcrp and Pgp are inhibited. Wild-type FVB mice were predosed intravenously with vehicle, Ko143 (10 mg/kg), GF120918 (10 mg/kg), and PSC833 (10 mg/kg) and then were dosed intravenously with digoxin (1 mg/kg) as described under Materials and Methods. Digoxin concentrations in blood, brain, and CSF were determined by LC/MS/MS. The impact of inhibiting Bcrp and Pgp on digoxin blood, brain, and CSF concentrations is shown in Fig. 8. Digoxin AUCs in the absence and presence of the inhibitors are summarized in Table 3. Digoxin blood concentrations increased up to 2-fold in the presence of the Pgp/Bcrp dual inhibitor GF120918 and 2- to 3-fold in the presence of the Pgp-specific inhibitor PSC833 (Fig. 8A). The increase was more significant from 30 min to 6 h after digoxin administration. Digoxin blood AUC increased approximately 2-fold in the presence of GF120918 and PSC833 (Table 3). Compared with the increase in blood concentration and AUC, more substantial increases were observed in digoxin brain and CSF concentrations and AUC. In the presence of GF120918 and PSC833, digoxin brain and CSF concentrations increased 4- to 6-fold and 4- to 8-fold, respectively (Fig. 8). Digoxin brain and CSF AUC increased 4- to 5-fold in the presence of PSC833 and 6- to 10-fold in the presence of GF120918 (Table 3). Digoxin brain/blood and CSF/blood AUC ratios increased 2- to 3-fold in the presence of GF120918 and 3- to 6-fold in the presence of PSC833 (Table 3). Because of the similar extent of increased exposures in brain and CSF, the change in digoxin CSF/brain AUC ratio was less than 1.5-fold in the presence of GF120918 and PSC833. Compared with GF120918 and PSC833, the Bcrp-specific inhibitor Ko143 had much less effects on digoxin exposures in blood, brain, and CSF (Fig. 8; Table 3). Digoxin exposures in blood and brain AUC decreased by approximately 18%, and digoxin exposures in CSF AUC increased by approximately 50%. Digoxin brain/blood, CSF/blood, and CSF/brain AUC ratios were not markedly affected in the presence of Ko143. The brain/blood ratio decreased slightly, and CSF/blood and CSF/brain ratios increased by 70 and 80%, respectively (Table 3).
Digoxin concentration time profiles of blood (A), brain (B), and CSF (C) in wild-type FVB mice after intravenous dosing of digoxin (1 mg/kg). Closed circles, concentrations in mice that were predosed intravenously with vehicle; open circles, concentrations in mice that were predosed intravenously with GF120918 (10 mg/kg); closed triangles, concentrations in mice that were predosed intravenously with Ko143 (10 mg/kg); open triangles, concentrations in mice that were predosed intravenously with PSC833 (10 mg/kg). Data are presented as mean ± S.D.
Digoxin AUC changes in wild-type mice predosed intravenously with vehicles or inhibitors
Digoxin blood, brain, and CSF AUC in wild-type mice in the absence and presence of the inhibitors were determined, and AUC ratios were calculated.
Discussion
The expression of both Bcrp and Pgp on brain microvessel endothelial cells has been demonstrated, and it has been shown that Bcrp and Pgp work in concert in regulating the penetration of drugs across the BBB (Chen et al., 2009; Polli et al., 2009; Kodaira et al., 2010). For the cosubstrates of both Bcrp and Pgp, however, the function of Bcrp in restricting drug brain penetration is often masked because of the more pronounced role played by Pgp at the BBB. The function of Bcrp can be observed only when the activities of both Bcrp and Pgp are disrupted either by the Pgp/Bcrp dual inhibitor GF120918 or in Mdr1a/1b(−/−)/Bcrp(−/−) mice (Breedveld et al., 2005; Bihorel et al., 2007; de Vries et al., 2007; Giri et al., 2008; Chen et al., 2009). Therefore, the identification and characterization of a Bcrp-selective substrate becomes necessary when investigating Bcrp function in regulating drug transport across the BBB, as well as evaluating Bcrp-mediated drug-drug interactions. The present study clearly demonstrated that dantrolene can be used as a Bcrp-selective substrate both in vitro and in vivo. In vitro studies using the Caco-2 cell system showed that dantrolene is a Bcrp-selective substrate, but not a Pgp substrate. Dantrolene exhibited greater B-A transport than A-B transport across Caco-2 monolayers. The transport was inhibited by the Bcrp specific inhibitor Ko143 or the Pgp/Bcrp dual inhibitor GF120918, but not the Pgp-specific inhibitor PSC833 (Fig. 1). Dantrolene is not only a selective substrate, but also a selective inhibitor of Bcrp over Pgp. Dantrolene exhibited significant inhibition on Bcrp-mediated transport of estrone-3-sulfate, but only slight inhibition on Pgp-mediated transport of digoxin (Fig. 2). Dantrolene as a Bcrp-selective substrate was then evaluated in vivo by knocking out Bcrp and Mdr1a/1b genes or by inhibiting Bcrp and Pgp functions with chemical inhibitors. In vivo studies using Bcrp(−/−), Mdr1a/1b(−/−), and Mdr1a/1b(−/−)/Bcrp(−/−) mice demonstrated that dantrolene brain and CSF exposures were significantly increased by knocking out Bcrp. In contrast, knocking out the Mdr1a/1b gene alone had no significant impact on the brain penetration of dantrolene (Figs. 3⇑–5; Table 1). The same conclusion was drawn by determining the dantrolene penetration across the BBB in the presence of Pgp and Bcrp inhibitors. In vivo studies with chemical inhibitors indicated only the Bcrp-specific inhibitor Ko143 or the Pgp/Bcrp dual inhibitor GF120918, but not the Pgp-specific inhibitor PSC833, increased dantrolene exposures in brain and CSF (Figs. 5⇑–7; Table 2). The fact that the Pgp/Bcrp dual inhibitor GF120918 exhibited an effect similar to that of the Bcrp-specific inhibitor Ko143 on dantrolene BBB penetration further supported that dantrolene BBB penetration was predominantly determined by Bcrp. Results from this study are consistent with results published by Enokizono et al. (2008) and Kodaira et al. (2010).
The function of Pgp in restricting digoxin transport across the BBB was also demonstrated in the study. Results from the present study indicated digoxin brain and CSF exposures were significantly increased in the presence of the Pgp inhibitor PSC833 or the Pgp/Bcrp dual inhibitor GF120918, but not the Bcrp-specific inhibitor Ko143 (Fig. 8; Table 3). Unlike dantrolene, digoxin blood exposures also increased in the presence of the Pgp inhibitors. The increased blood exposure of digoxin was most likely due to the reduced renal secretion, as it has been shown that Pgp at the luminal membrane of the renal tubule is involved in digoxin renal elimination (Hori et al., 1993; Okamura et al., 1993; Shoaf et al., 2011).
There are two major pathways to deliver drug to CSF, namely transport from brain to CSF by crossing the ependymal cell monolayers between the brain and CSF interface or transport from blood to CSF by crossing the BCSFB. Most studies have suggested that brain to CSF transport is the major route of drug delivery; therefore, CSF concentration can be used as a surrogate to assess the free drug concentration in brain tissues (Liu et al., 2006, 2009; Lin, 2008). Compared with the knowledge of the effect of Bcrp and Pgp in restricting drug transport across the BBB, little is known about their impact on drug transport across the BCSFB. Most studies indicated Pgp, but not Bcrp, is expressed on the luminal membrane of the choroid plexus epithelial cells (Rao et al., 1999; Daood et al., 2008). Because Pgp is localized to the luminal membrane of choroid plexus facing the CSF lumen instead of the blood vessel, it is anticipated that inhibiting Pgp will increase the CSF concentrations of Pgp substrates. Although it has been demonstrated that inhibiting Bcrp and Pgp increases the brain exposures of Bcrp and Pgp substrates, it is still not clear whether and how the exposures in CSF will be affected when Bcrp and Pgp are inhibited. Limited results showed that the CSF exposures of Pgp substrates could be decreased, increased, or not affected when Pgp was blocked (Doran et al., 2005; Zhuang et al., 2006; Mealey et al., 2008). Because the brain exposures of Bcrp and Pgp substrates are increased when Bcrp and Pgp are inhibited, it then becomes a question of how the CSF exposures will change and whether the changes in CSF exposures can reflect the change in brain exposures when Bcrp and Pgp are inhibited. To answer the question, the influence of Bcrp/Pgp on Bcrp/Pgp substrate exposure in blood, brain, and CSF was investigated in the present study. To rule out the effects from other transporters, Bcrp- and Pgp-selective probe substrates and specific inhibitors were selected in the study. The selectivity of dantrolene to Bcrp was reported previously (Enokizono et al., 2008; Kodaira et al., 2010) and was further confirmed in the present study in vitro using the Caco-2 cell system as well as in vivo using both chemical inhibitors and gene knockout mice. For both dantrolene and digoxin, results from the present study indicated that blocking Bcrp or Pgp increased not only the exposures in brain, but also the exposures in CSF. More importantly, dantrolene and digoxin exposures in CSF increased to an extent similar to that in brain when Bcrp and Pgp were inhibited. Therefore, whereas inhibiting Bcrp and Pgp increased the exposures in both brain and CSF, it had little impact on the CSF/brain ratios. Compared with the profound increase on brain/blood and CSF/blood AUC ratio, the changes of CSF/brain AUC ratio of both dantrolene and digoxin were insignificant. Dantrolene CSF/brain AUC ratio changed less than 1.5-fold in Bcrp and Mdr1a/1b gene knockout mice or in the presence of the Pgp and Bcrp inhibitors (Tables 1 and 2). The CSF/brain ratio of digoxin changed less than 2-fold in the presence of Pgp and Bcrp inhibitors (Table 3). The results suggested that there are rapid equilibrium and/or transport of dantrolene and digoxin from brain to CSF, and the brain to CSF transport plays a major role in delivering dantrolene and digoxin to CSF.
In summary, the present study evaluated the selectivity of dantrolene to Bcrp and investigated the effect of Bcrp and Pgp in determining drug distribution in brain and CSF. Results from the study demonstrated that dantrolene is a selective substrate of Bcrp, and CSF exposures of Pgp and BCRP substrates are primarily determined by the transport from brain to CSF. Because of the leaky ependymal cell monolayer between brain and CSF interface and the tight BCSFB between blood and CSF (Johanson et al., 2008), it is likely that the transport from brain to CSF across ependymal cell monolayer is more significant than the transport from blood to CSF across the BCSFB, especially for compounds with low to moderate permeability. Therefore, blocking Bcrp and Pgp increases not only the exposures in brain but also the exposures in CSF to a similar extent. Although it could be compound-dependent, the present study suggests that for Pgp and BCRP substrates, blocking Pgp and BCRP has a slight impact on the CSF/brain exposure ratio; therefore, CSF can be used as a surrogate to assess their exposures in brain.
Authorship Contributions
Participated in research design: Xiao, Rohde, and Gan.
Conducted experiments: Black, Hetu, Sands, Wang, and Caputo.
Wrote or contributed to the writing of the manuscript: Xiao, Black, Sands, and Gan.
Footnotes
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
ABBREVIATIONS:
- Pgp
- P-glycoprotein
- BBB
- blood-brain barrier
- Bcrp
- breast cancer resistance protein
- BCSFB
- blood cerebrospinal fluid barrier
- CSF
- cerebrospinal fluid
- PSC833
- 6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-l-valine-cyclosporine A
- Ko143
- (3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester
- GF120918
- N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide
- CPDPX
- 8-cyclopently-1,3-dipropylxanthine
- LC/MS/MS
- liquid chromatography/tandem mass spectrometry
- AUC
- area under the curve
- A-B
- apical-to-basolateral
- B-A
- basolateral-to-apical
- MK571
- (E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid.
- Received November 5, 2011.
- Accepted January 19, 2012.
- Copyright © 2012 by The American Society for Pharmacology and Experimental Therapeutics