Abstract
This study was designed to evaluate the use of cerebrospinal fluid (CSF) drug concentration and plasma unbound concentration (Cu,plasma) to predict brain unbound concentration (Cu,brain). The concentration-time profiles in CSF, plasma, and brain of seven model compounds were determined after subcutaneous administration in rats. The Cu,brain was estimated from the product of total brain concentrations and unbound fractions, which were determined using brain tissue slice and brain homogenate methods. For theobromine, theophylline, caffeine, fluoxetine, and propranolol, which represent rapid brain penetration compounds with a simple diffusion mechanism, the ratios of the area under the curve of Cu,brain/CCSF and Cu,brain/Cu,plasma were 0.27 to 1.5 and 0.29 to 2.1, respectively, using the brain slice method, and were 0.27 to 2.9 and 0.36 to 3.9, respectively, using the brain homogenate method. A P-glycoprotein substrate, CP-141938 (methoxy-3-[(2-phenyl-piperadinyl-3-amino)-methyl]-phenyl-N-methyl-methane-sulfonamide), had Cu,brain/CCSF and Cu,brain/Cu,plasma ratios of 0.57 and 0.066, using the brain slice method, and 1.1 and 0.13, using the brain homogenate method, respectively. The slow brain-penetrating compound, N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl-]sarcosine, had Cu,brain/CCSF and Cu,brain/Cu,plasma ratios of 0.94 and 0.12 using the brain slice method and 0.15 and 0.018 using the brain homogenate method, respectively. Therefore, for quick brain penetration with simple diffusion mechanism compounds, CCSF and Cu,plasma represent Cu,brain equally well; for efflux substrates or slow brain penetration compounds, CCSF appears to be equivalent to or more accurate than Cu,plasma to represent Cu,brain. Thus, we hypothesize that CCSF is equivalent to or better than Cu,plasma to predict Cu,brain. This hypothesis is supported by the literature data.
It is a commonly accepted assumption that unbound or free drug is the species available for interaction with drug targets within the body, and this is referred to as the free drug hypothesis. For drugs with an intended action in the central nervous system (CNS), it is assumed that unbound drug in interstitial spaces in the brain (Cu,brain) is in direct contact or in equilibrium with the site of action (de Lange and Danhof, 2002). Therefore, in preclinical and clinical pharmacokinetic/pharmacodynamic studies, it is critical to determine Cu,brain for brain-targeted compounds. In preclinical pharmacokinetic/pharmacodynamic studies, to prove the mechanism of action or to validate an in vivo efficacy model, it is necessary to demonstrate the correlation between the affinity (e.g., Ki or IC50) determined from in vitro pharmacology assays and the in vivo efficacious drug concentration at the site of action. Furthermore, in phase I clinical trials, it is important to determine whether a safe dose is observed that will result in sufficient Cu,brain to demonstrate efficacy in phase II trials. Because the brain is separated from the systemic circulation by the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB), it has been a challenge to directly estimate Cu,brain. Microdialysis has been used to measure Cu,brain, but this method is resource-demanding and not applicable to compounds with particular physicochemical properties. Specifically, many compounds in the discovery stage of testing are often very lipophilic, and it has been difficult to apply microdialysis to study these compounds because of high nonspecific binding and poor recovery (Carneheim and Stahle, 1991; Khramov and Stenken, 1999; Lindberger et al., 2002). In clinical trials, brain microdialysis cannot be readily used, due to ethical reasons, except in special circumstances (Scheyer et al., 1994a,b; Joukhadar et al., 2001; Hillered et al., 2005). Other noninvasive imaging technologies, such as positron emission tomography, have been used to study mechanism of action, such as receptor occupancy in brain tissue. However, the imaging ligands for many novel drug targets may not be available or cannot be developed quickly for decision making during clinical trials (Cunningham et al., 2004).
Two surrogate approaches, namely, plasma unbound concentration (Cu,plasma) and CSF concentration (CCSF), have been used to estimate the Cu,brain indirectly. Obviously, due to efflux transporters at the BBB, Cu,plasma may not be equal to Cu,brain (Liu and Chen, 2005). Data from brain microdialysis indicate that Cu,plasma is higher than Cu,brain for many of the compounds that have been studied (Hammarlund-Udenaes et al., 1997; Sawchuk and Elmquist, 2000). Because CSF is in direct contact with the brain tissue, it is assumed to readily equilibrate with brain interstitial fluid concentration (Meineke et al., 2002; Shen et al., 2004). CSF has been used as a common surrogate measure for Cu,brain in clinical pharmacology studies (Bonati et al., 1982; Cherubin et al., 1989; Garver, 1989; Reiter and Doron, 1996; Ostermann et al., 2004). Nevertheless, the value of measuring CCSF has been challenged (Bonati et al., 1982; de Lange and Danhof, 2002). Studies in the last decade using molecular biology approaches have demonstrated that the expression of many transporters at the BCSFB is different from that at the BBB, supporting the opinion that the CSF drug concentration can significantly deviate from Cu,brain (Kusuhara and Sugiyama, 2004).
Although numerous studies have been conducted to examine the relationship between CCSF, Cu,plasma, and Cu,brain, no studies have been conducted to compare CCSF and Cu,plasma in prediction of Cu,brain. The objective of this work was to examine the accuracy of using CCSF and Cu,plasma to predict Cu,brain. It was recognized that generalizations like this could have serious pitfalls. However, we hoped to identify situations where these empirical observations are reasonable to facilitate the drug discovery and development process for CNS-targeted agents.
Materials and Methods
Chemicals. Caffeine, fluoxetine, propranolol, theobromine, and theophylline were obtained from Sigma-Aldrich (St. Louis, MO). N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) and methoxy-3-[(2-phenyl-piperadinyl-3-amino)-methyl]-phenyl-N-methyl-methane-sulfonamide (CP-141938) were synthesized at Pfizer Global Research and Development Laboratories (Groton, CT) with purity greater than 98%. All other chemicals used in the experiments were of the highest available grade.
Animal Experiments. Male Sprague-Dawley rats (250–280 g) were obtained from Charles River (Raleigh, NC). They were housed at controlled temperature and humidity in an alternating 12-h light and dark cycle with free access to food and water. Rats received caffeine (10 mg/kg), CP-141938 (5 mg/kg), fluoxetine (10 mg/kg), NFPS (10 mg/kg), propranolol (5 mg/kg), theobromine (10 mg/kg), or theophylline (10 mg/kg) subcutaneously. The doses were prepared in 0.9% saline and delivered in a volume of 2 ml/kg. After the animals were sacrificed in a CO2 chamber, approximately 50-μl CSF samples were collected at designated times between 10 min and 24 h postdose via cisterna magna puncture and were stored at –20°C before analysis. In the same study, blood and brain samples were collected, and the results were published previously (Liu et al., 2005).
Sample Analysis. Twenty microliters of CSF and acetonitrile were mixed in silanized 96-well glass tubes. The HPLC-tandem mass spectrometry system consisted of either a Shimadzu ternary pump (LC-10A; Shimadzu, Kyoto, Japan) or an Agilent quaternary pump HPLC system (Hewlett Packard, Palo Alto, CA), an HTS-PAL autosampler (Leap Technologies, Zwingen, Switzerland) and a PE Sciex API 3000 or 4000 (Perkin-Elmer Sciex Instruments, Foster City, CA) mass spectrometer with a turbo ion spray interface (PE-Sciex, Thornhill, ON, Canada). A 10-μl aliquot of each sample was injected onto a HPLC column. The HPLC-tandem mass spectrometry methods of the seven model compounds in plasma and brain samples have been described previously (Liu et al., 2005). The same methods were used for the CSF samples. The low limits of quantitation for caffeine, CP-141938, fluoxetine, NFPS, propranolol, theobromine, and theophylline were 1.0, 0.50, 2.5, 0.20, 0.5, 50, and 50 ng/ml, respectively. The assay accuracy was between 80% and 120%.
Data Analysis. The plasma free concentrations were calculated from the product of the plasma unbound fraction and plasma concentration. The brain free concentrations were calculated from the product of the brain unbound fraction and brain concentration. The unbound fraction in plasma was determined using equilibrium dialysis. The unbound fraction in brain was determined using brain homogenate and brain tissue slice method as described previously (Liu et al., 2005; Becker and Liu, 2006). Briefly, for the brain homogenate method, brain tissue was homogenized in 2 volumes of buffer. The brain homogenate was spiked with a compound and incubated at 37°C for 5 h in an equilibrium dialysis apparatus. The unbound fractions determined in the diluted brain tissue homogenates were corrected to yield an estimate of unbound fraction in the intact brain tissue. For the brain slices method, brain slices of the cortex (400 μm) were prepared using a McIlwain Tissue Chopper. The brain slices and buffer were spiked with a compound and incubated at 37°C for 6 h.
The area under the curve from time 0 to infinity (AUC(0-∞)) was calculated using WinNonlin (version 3.2; Pharsight Corporation, Mountain View, CA) as the sum of the area from zero to the last time point using the trapezoidal rule, and the area from the last time point to infinity using the ratio of plasma concentration at the last time point and the slope of the terminal phase.
Results
The compounds selected for evaluation included a range of physicochemical properties, BBB permeability, brain disposition profiles, and efflux transporter activity, and this information was presented in our previous work (Liu et al., 2004, 2005). The P-gp transport activity, the BBB permeability (quantified as permeability-surface area product, PS), unbound fractions in plasma and brain tissue, the AUC of plasma, CSF, and brain tissue concentrations, and the AUC ratios of Cu,brain/CCSF, Cu,brain/Cu,plasma, and CCSF/Cu,plasma are listed in Table 1. As shown in Table 1, the compounds selected represent weak or nonsubstrates as well as moderate to strong P-gp substrates.
P-gp activities, unbound fractions, AUC, and AUC ratios of seven model compounds
Relationship between Cu,brain and CCSF. For theobromine, theophylline, caffeine, fluoxetine, and propranolol, the time course of Cu,brain/CCSF increased over time and reached a plateau in less than 0.5 h postdose for all compounds except fluoxetine, which reached a plateau 1 h postdose (data not shown). The AUC ratios of Cu,brain/CCSF were between 0.27 and 1.5 using the brain unbound fraction determined from brain slices and between 0.27 and 2.9 using the brain unbound fraction determined from brain homogenate. For CP-141938, a P-gp substrate, its CSF concentrations equilibrated with brain concentrations in 0.5 h. Its Cu,brain/CCSF ratios were 0.57 and 1.1 using the brain unbound fraction determined from brain slices and the brain homogenate method, respectively. The weak P-gp substrate, NFPS, did not reach equilibrium between CSF and brain up to 24 h postdose (data not shown). The NFPS AUC ratios of Cu,brain/CCSF were 0.94 and 0.15 using unbound fraction from brain slices and brain homogenate methods, respectively (Table 1). Thus, for the rapid brain penetration compounds that were studied, CCSF is similar to Cu,brain. NFPS was the example of a slow brain penetration compound, and in this case, CCSF was similar to Cu,brain when based on the unbound fraction in brain tissue slices, but CCSF was greater than Cu,brain when based on the unbound fraction obtained using the brain homogenate method (Fig. 1).
Relationship between Cu,brain and Cu,plasma. Like CSF, drug concentrations in plasma equilibrated rapidly with brain for theobromine, theophylline, caffeine, fluoxetine, and propranolol (data not shown). The AUC ratios of Cu,brain/Cu,plasma for the five compounds were between 0.29 and 2.1 using the brain unbound fraction determined from brain slices and between 0.36 and 3.9 using the brain unbound fraction determined from brain homogenate. The concentration of CP-141938 in plasma equilibrated with brain concentration by 0.5 h. Its AUC ratios of Cu,brain/Cu,plasma were 0.066 and 0.13 using the unbound fraction determined from brain slices and brain homogenate, respectively. For NFPS, a weak P-gp substrate, its Cu,plasma did not equilibrate with brain concentrations up to 24 h postdose. The Cu,brain/Cu,plasma for NFPS was 0.12 using the brain unbound fraction determined from brain slices (Table 1). When the brain-unbound fraction determined from brain homogenate was used, the ratio was 0.018. These data indicate that the Cu,plasma is close to Cu,brain for all the compounds except CP-141938 and NFPS. The Cu,plasma values of CP-141938 and NFPS were much greater than their Cu,brain values (Fig. 1).
AUC ratio of Cu,brain/Cu,plasma (solid bars) and Cu,brain/CCSF (open bars) of seven model compounds in rats. The data were from Table 1. The solid and broken lines represent unity and 3-fold boundaries, respectively. The brain unbound fractions in A and B were determined using the brain slice method and homogenate method, respectively.
Relationship between CCSF and Cu,plasma. For all seven compounds, the CSF and plasma concentrations showed similar terminal half-lives (data not shown). The time course of CCSF/Cu,plasma ratio increased over time and reached a plateau rapidly. For theobromine, theophylline, caffeine, fluoxetine, and propranolol, the AUC ratios of CCSF/Cu,plasma were between 0.7 and 1.4. For CP-141938 and NFPS, their CCSF/Cu,plasma ratios were 0.11 and 0.13, respectively (Table 1).
Discussion
The seven model compounds were selected because their physicochemical properties, brain disposition, binding properties, and transport properties have been well characterized in our previous study (Liu et al., 2005). The unbound concentrations were considered similar if their values were within 3-fold. This criterion was chosen to allow for differences due to experimental error and for actual differences that would be considered to have significant pharmacological consequences (Maurer et al., 2005).
On the basis of the results from this study, we hypothesize that although CCSF may not necessarily be equal to Cu,brain, it is equivalent to or better than Cu,plasma to predict Cu,brain. For five of the seven model compounds in the present study, their Cu,plasma was within 3-fold of their Cu,brain. For the other two compounds, their Cu,plasma was 15- to 56-fold of their Cu,brain. In contrast, the CCSF was within 3-fold of the Cu,brain for the seven compounds except NFPS. The CCSF of NFPS was similar to the Cu,brain using the unbound fraction determined with the brain slice method and was 6.7-fold of the Cu,brain using the unbound fraction determined with the brain homogenate method. This discrepancy was due to the fact that the unbound fraction in brain slice was approximately 7-fold greater than the value measured using brain homogenate. The lower brain unbound fraction measured using the brain homogenate method was probably caused by greater access to the binding sites that are normally inaccessible to a compound in the intact brain tissue. This view is consistent with our previous data in that, using the brain slices method, the predicted rat brain to plasma ratio was within 4-fold of the observed in vivo value, but using the brain homogenate method, the predicted brain to plasma ratio was 27-fold greater than the observed one (Becker and Liu, 2006). The NFPS data from the present and previous studies indicate that the brain slice method is more accurate than the brain homogenate method to determine brain unbound fraction. More studies are needed to confirm this observation.
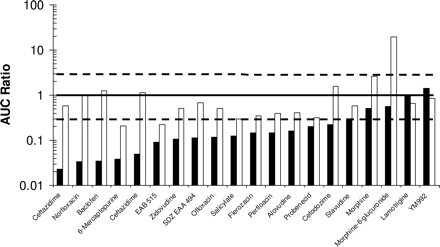
The plot of Cu,brain/Cu,plasma (solid bars) and Cu,brain/CCSF (open bars) of 20 compounds in rats or rabbits. Cu,brain represents brain interstitial drug concentrations determined using microdialysis. The concentrations were the values observed at steady state or as AUC after single administration. The data were modified from Shen et al. (2004). The solid and broken lines represent unity and 3-fold boundaries, respectively.
Our hypothesis is supported by the data in the literature. Shen et al. (2004) compiled a data set generated from preclinical animals for 20 compounds, in which the Cu,brain was determined using microdialysis as the interstitial drug concentrations. Figure 2 supports the view that CCSF is closer to Cu,brain than Cu,plasma for all the compounds except for morphine-6-glucuronide. The CCSF of morphine-6-glucuronide is significantly greater (19-fold) than Cu,brain, but Cu,plasma is similar to Cu,brain (Stain-Texier et al., 1999). These results may be due to the CSF sink effect since morphine-6-glucuronide has low BBB permeability or to different transporters at the BBB and BCSFB. If this compound is excluded from the data set, the Cu,brain/CCSF and Cu,brain/Cu,plasma values (mean ± S.D.) are 0.74 ± 0.58 and 0.26 ± 0.37 (n = 19), respectively. Our results are also consistent with a recent study in mice by Maurer et al. (2005) in which the brain unbound fraction was determined using the brain homogenate method. For 22 of 33 CNS drugs, their CCSF, Cu,plasma, and Cu,brain exhibited similar values. For four drugs, 9-hydroxyrisperidone, risperidone, sulpiride, and thiopental, CCSF is more accurate than Cu,plasma in prediction of Cu,brain. Only for two drugs, buspirone and caffeine, the CCSF deviates significantly from Cu,brain when compared with the corresponding Cu,plasma. However, caffeine in the present study clearly demonstrated identical concentrations for CCSF, Cu,plasma, and Cu,brain in rats.
It is interesting to note that the ratio of Cu,brain/CCSF is less than unity for most compounds in the present study and in the literature, as well (Fig. 2). Because CSF protein concentrations are normally 0.5% or less of the respective plasma concentrations, it has been assumed that the protein binding in CSF is negligible in most CSF studies (Lin and Lu, 1997). This assumption may lead to an underestimation of the true ratio of Cu,brain/CCSF for extensive protein-binding compounds since the protein binding can be significant in CSF for lipophilic amines (Nyberg et al., 1981; Wode-Helgodt and Alfredsson, 1981). Shen at al. (2004) have proposed that the unbound fraction of drug in CSF needs to be determined to more accurately assess drug concentrations at the biophase in the CNS. Further studies are needed to examine whether the Cu,brain/CCSF ratios are closer to unity if the protein binding in the CSF is considered.
The seven model compounds can be empirically divided into two classes with the following characteristics: class I compounds, Cu,plasma ≈ CCSF ≈ Cu,brain; and class II compounds: Cu,plasma > CCSF ≥ Cu,brain. Class I compounds include theobromine, theophylline, caffeine, fluoxetine, and propranolol. Compounds in Class I penetrate the brain quickly and are presumed to cross the BBB and BCSFB via passive diffusion. Class II compounds include CP-141938 and NFPS. CP-141938 penetrates the brain quickly but is subject to significant P-gp efflux. Its brain to plasma ratio in mdr1a/b knockout mice was 50-fold of that in wild-type mice (Smith et al., 2001). At equilibrium, the CCSF of CP-141938 is similar to its Cu,brain, but its Cu,plasma is significantly higher than its Cu,brain. NFPS is a weak P-gp substrate and penetrates the brain slowly. Based on unbound fraction from brain slices, CCSF predicts Cu,brain and Cu,plasma overpredicts Cu,brain; based on unbound fraction from brain homogenate, both CCSF and Cu,plasma overpredict Cu,brain, but CCSF is closer to Cu,brain than Cu,plasma. We hypothesize that for class I compounds, which penetrate the brain quickly via passive diffusion, CCSF and Cu,plasma show similar accuracy to predict Cu,brain; for class II compounds, which penetrate the brain slowly or have high efflux activities, CCSF appears to be similar to or better than Cu,plasma to predict Cu,brain, although CCSF may not equal Cu,brain.
Although only two class II compounds were in the present study, our hypothesis is supported by the results from Maurer et al., (2005). In that study, three compounds, metocloperamide, risperidone, and 9-hydroxyrisperidone were shown as P-gp substrates with brain/plasma concentration ratios in the P-gp knockout versus P-gp competent mice of 6.6, 10, and 17, respectively. Therefore, these compounds fit into the class II category. Based on our hypothesis, we would expect that CCSF will be equal to or better than Cu,plasma to predict Cu,brain for these compounds. Indeed, this prediction is consistent with the observed data. We calculated the Cu,brain/Cu,plasma and Cu,brain/CCSF ratios based on the total concentration ratios and binding in plasma and brain tissue homogenate. The Cu,brain/Cu,plasma are 0.52, 0.26, and 0.02, and Cu,brain/CCSF ratios are 0.86, 2.7, and 0.12, respectively, in the P-gp-competent mice. These data demonstrated that the CCSF concentration is similar to the Cu,plasma to predict Cu,brain for metoclopramide and risperidone but better than Cu,plasma for 9-hydroxyrisperidone, although its CCSF still overpredicts the Cu,brain. Furthermore, we anticipate that the advantage of using CCSF to predict Cu,brain for P-gp substrates in P-gp-competent mice will be diminished in P-gp knockout mice because these P-gp substrates become class I compounds in P-gp knockout mice. As expected, Cu,brain/Cu,plasma ratios (3.5, 2.6, and 0.26) were similar to those of Cu,brain/CCSF (2.6, 4.1, and 0.20) for metocloperamide, risperidone, and 9-hydroxyrisperidone, respectively.
Our hypothesis implies that P-gp has a more significant impact on the Cu,brain/Cu,plasma than on Cu,brain/CCSF ratios. This prediction is in good agreement with the data reported by Doran et al. (2005). The brain/plasma concentration ratios of P-gp substrates loperamide, verapamil, and quinidine in the P-gp knockout mice versus P-gp-competent mice were 9.3, 17, and 36, respectively, which were significantly greater than 3-fold. However, their brain/CSF concentration ratios in the P-gp knockout versus P-gp-competent mice were 1.5, 1.9, and 3.6, which were within or close to 3-fold.
Regardless of whether a compound belongs to class I or II, CCSF may be used as a surrogate estimate for Cu,brain in drug discovery settings because CSF samples can be readily collected during in vivo pharmacology screens from rodents, and only a single analytical assay is needed to estimate Cu,brain. For using Cu,plasma to predict Cu,brain, two assays, total plasma concentration and protein binding, are required to estimate brain unbound concentration. Unlike in animal studies, in clinical trials CSF collection adds substantial cost and increases the complexity of study design. For a class I compound that has been characterized in preclinical study, if no in vitro data suggest that it is a BBB efflux transporter substrate, CCSF may provide no more information than Cu,plasma. For a class II compound, it may be valuable to determine CCSF because it may more closely represent Cu,brain than Cu,plasma. By comparing the relationship between CCSF and Cu,plasma in humans and the relationship among CCSF, Cu,plasma, and Cu,brain in animals, we may be able to estimate Cu,brain in humans assuming no significant species difference in CNS drug disposition. Other approaches, such as imaging analysis, may be needed to verify the estimation. This view is consistent with that of Shen et al. (2004), that CSF penetration studies in animals can serve as predictive models in human drug development, even for compounds that are substrates of transporters at the BBB and BCSFB. This proposed classification system according to the characteristics of drug disposition in the CNS was based on a limited number of compounds and should be considered as a hypothesis. More studies are needed to further confirm this hypothesis.
In summary, the results in the present study indicate that although CCSF is equivalent to Cu,plasma as a surrogate measurement for Cu,brain for rapid brain penetration compounds that cross the BBB and BCSFB by passive diffusion, CCSF is more predictive than Cu,plasma for efflux transporter substrates or for slow brain-penetrating compounds. It is valuable to determine CCSF in drug discovery and possibly in some circumstances in clinical drug development
Acknowledgments
We thank Dr. Jae Lee for support of this project.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.008201.
-
ABBREVIATIONS: CNS, central nervous system; BBB, blood-brain barrier; CSF, cerebrospinal fluid; BCSFB, blood-CSF barrier; P-gp, P-glycoprotein; CCSF, CSF drug concentration; Cu,plasma, plasma unbound drug concentration; Cu,brain, brain unbound drug concentration; AUC, area under the curve; NFPS, N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine; CP-141938, methoxy-3-[(2-phenyl-piperadinyl-3-amino)-methyl]-phenyl-N-methyl-methane-sulfonamide; HPLC, high-performance liquid chromatography.
- Received November 4, 2005.
- Accepted May 23, 2006.
- The American Society for Pharmacology and Experimental Therapeutics