Abstract
3′-Hydroxy-4′-methoxydiclofenac (VI) is a human-specific metabolite known to accumulate in the plasma of patients after repeated administration of diclofenac sodium. Diclofenac also produces glutathione-conjugated metabolites, some of which are human-specific. In the present study, we investigated whether these metabolites could be generated in humanized chimeric mice produced from TK-NOG mice. After a single oral administration of diclofenac to humanized mice, the unchanged drug in plasma peaked at 0.25 hour and then declined with a half-life (t1/2) of 2.4 hours. 4′-Hydroxydiclofenac (II) and 3′-hydroxydiclofenac also peaked at 0.25 hour and were undetectable within 24 hours. However, VI peaked at 8 hours and declined with a t1/2 of 13 hours. When diclofenac was given once per day, peak and trough levels of VI reached plateau within 3 days. Studies with administration of II suggested VI was generated via II as an intermediate. Among six reported glutathione-conjugated metabolites of diclofenac, M1 (5-hydroxy-4-(glutathion-S-yl)diclofenac) to M6 (2′-(glutathion-S-yl)monoclofenac), we found three dichlorinated conjugates [M1, M2 (4′-hydroxy-3′-(glutathion-S-yl)diclofenac), and M3 (5-hydroxy-6-(glutathion-S-yl)diclofenac)], and a single monochlorinated conjugate [M4 (2′-hydroxy-3′-(glutathion-S-yl)monoclofenac) or M5 (4′-hydroxy-2′-(glutathion-S-yl)monoclofenac)], in the bile of humanized chimeric mice. M4 and M5 are positional isomers and have been previously reported as human-specific in vitro metabolites likely generated via arene oxide and quinone imine–type intermediates, respectively. The biliary monochlorinated metabolite exhibited the same mass spectrum as those of M4 and M5, and we discuss whether this conjugate corresponded to M4 or M5. Overall, humanized TK-NOG chimeric mice were considered to be a functional tool for the study of drug metabolism of diclofenac in humans.
Introduction
Diclofenac sodium was the first approved nonsteroidal anti-inflammatory drug, and has been widely used to treat pain or inflammation caused by arthritis or ankylosing spondylitis (Small, 1989). Metabolism studies in animals and humans revealed that this drug undergoes almost complete biotransformation by direct conjugation or oxidation of the aromatic rings followed by conjugation (Stierlin et al., 1979; Stierlin and Faigle, 1979). In humans, 4′-hydroxydiclofenac (II), 5-hydroxydiclofenac, 3′-hydroxydiclofenac (IV), and 4′,5-dihydroxydiclofenac are excreted into urine as their free (minor) or conjugated forms. While analyses on the concentrations of these metabolites in plasma samples from study subjects were performed, a new metabolite, 3′-hydroxy-4′-methoxydiclofenac (VI), was found. This metabolite showed distinct pharmacokinetic behavior with a time to maximum plasma concentration (Tmax) and half-life (t1/2) of 12 and 80 hours, respectively, after single oral administration with potential accumulation after repeated dosing of diclofenac (Degen et al., 1988; Faigle et al., 1988). According to Faigle et al. (1988), unpublished data showed that VI was not found in mouse, rat, or marmoset plasma, but baboon plasma only. The cumulative urinary excretion (0–96 hours) of free and conjugated VI in humans was very small (ca. 1% of dose), which might explain why VI had not been found in the urine sample in the previous studies.
Formation of a reactive 1-O-acyl glucuronide by direct glucuronidation of diclofenac is considered to be responsible for the major modification of proteins (Kretz-Rommel and Boelsterli, 1993). Evidence of this includes the detection of diclofenac-S-acyl-glutathione in rat bile treated with high doses of diclofenac (Grillo et al., 2003). The formation of reactive metabolites of diclofenac by various cytochrome P450s (P450s), followed by their glutathione-dependent inactivation, has been previously studied (Boelsterli, 2003; Tang, 2003). Tang et al. (1999) identified three glutathione conjugates—namely, 5-hydroxy-4-(glutathion-S-yl)diclofenac (M1), 4′-hydroxy-3′-(glutathion-S-yl)diclofenac (M2), and 5-hydroxy-6-(glutathion-S-yl)diclofenac (M3)—in rat bile and with in vitro incubations in rat liver microsomes or human hepatocytes. This suggested the formation of quinone imine intermediates during diclofenac metabolism. On the other hand, a novel glutathione conjugate, 2′-hydroxy-3′-(glutathion-S-yl)monoclofenac (M4), was reported to be generated by incubation of diclofenac with human liver microsomes, suggesting the existence of an arene oxide intermediate as another route of bioactivation (Yan et al., 2005). Experiments using recombinant enzymes and specific inhibitors demonstrated that CYP2C9 was involved in the formation of M4, and this metabolite was not detected in incubations with either rat or monkey liver microsomes. Around the same time, Yu et al. (2005) reported another monochlorinated glutathione conjugate with the same molecular weight, 4′-hydroxy-2′-(glutathion-S-yl)monoclofenac (M5), followed by the discovery of the latest glutathione conjugate, 2′-(glutathion-S-yl)monoclofenac (M6) (Wen et al., 2008; Boerma et al., 2012). Although M4, M5, and M6 are generated in in vitro studies using recombinant enzymes or liver microsomes fortified with glutathione, in vivo formation of these metabolites has not been previously reported.
Chimeric mice with humanized livers hold considerable advantages for more accurate prediction of human metabolism over conventional in vivo animal or in vitro human systems (Yoshizato et al., 2012; Grompe and Strom, 2013; Peltz, 2013). The ability to predict circulating human metabolites is one of the characteristic features of this model which could satisfy regulatory requirements for the safety testing of drug metabolites (Kamimura et al., 2010). Histologic examination showing the connection of human bile canaliculi to a mouse interlobular bile duct suggested easy access to human bile samples, unlike in clinical experiments (Kamimura et al., 2010). Following the development of immunodeficient humanized mice with a urokinase-type plasminogen activator (uPA) transgene (Mercer et al., 2001) or a disrupted gene encoding murine fumarylacetoacetate hydrolase (Azuma et al., 2007), NOD/Shi-scid IL2 receptor gamma–null mice expressing a herpes simplex virus type-1 thymidine kinase transgene (TK-NOG mice) with humanized liver were generated (Hasegawa et al., 2011). Liver-specific expression of thymidine kinase by the albumin enhancer/promoter results in damage to murine hepatocytes following administrations of a nontoxic dose of ganciclovir (GCV). This new humanized mouse model has demonstrated formation of the human metabolites of debrisoquine (Hasegawa et al., 2011), thalidomide including a glutathione conjugate of its 5-hydroxy form in plasma (Yamazaki et al., 2012), and clemizole (Nishimura et al., 2013). These results, the application of this model in pharmacokinetic modeling (Tsukada et al., 2013; Suemizu et al., 2014; Yamashita et al., 2014), and the evaluation of drug-drug interactions (Nishimura et al., 2013; Yamazaki et al., 2013) prompted us to conduct diclofenac metabolism studies using humanized TK-NOG mice. In this report, the formation of the human-specific metabolites IV, VI, and glutathione conjugate metabolites is presented. Additionally, administration of a synthetic version of metabolite II was conducted to elucidate the pathways of diclofenac metabolism in humans.
Materials and Methods
Diclofenac sodium [2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid sodium salt] was purchased from UPS (Rockville, MD). 4′-Hydroxydiclofenac (synthetic version of metabolite II) was purchased from Becton, Dickinson and Company (Franklin Lakes, NJ). [13C6]4′-Hydroxydiclofenac, used as an internal standard, was obtained from Toronto Research Chemicals (Ontario, Canada). Other chemicals and solvents were of analytical grade. Hepatocytes from a 4-year-old female donor (lot 7F3063; LONZA, Japan, Tokyo) or those from a 9-year old female donor (lot HC2-19; XenoTech, Lenexa, KS) were used for the transplantation. Metabolic activities of CYP2C9 in the respective hepatocytes were 1.28 (4′-hydroxylation of tolbutamide at 400 μM) or 467 (4′-hydroxylation of diclofenac at 100 μM) pmol/min per 106 viable cells.
Reference incubation mixture containing M6 was prepared at VU University Amsterdam (Amsterdam, The Netherlands) as described by Boerma et al. (2014). In brief, a final concentration of 500 μM diclofenac was incubated with 100 nM horseradish peroxidase, 100 μM glutathione, and 8 μM human glutathione-S-transferase P1-1 for 1 hour at 37°C. The reaction was terminated by the addition of methanol.
Animals, Dosing, and Sample Collection.
The humanized TK-NOG chimeric mice were generated using the method of Hasegawa et al. (2011), with some modifications. For the plasma concentration studies, 11- or 12-week-old male TK-NOG mice were given intraperitoneal injections of 6 mg/kg GCV, and then 6 and 50 mg/kg GCV was given 2 and 10 days later, respectively. Eighteen days after the first GCV dose, mice with elevated serum alanine aminotransferase (ALT) levels of more than 200 IU/l received transplants of 1.1 or 1.5 × 106 of human hepatocytes by intrasplenic injection. For the biliary excretion studies, 8- or 10-week-old male TK-NOG mice were given intraperitoneal injections of 10 mg/kg GCV, and an additional 50 mg/kg GCV was given to a subset of these mice 8 days later. Eight to 17 days after the first dose, mice with serum ALT levels of more than 240 IU/l received transplants of 0.6 or 1.0 × 106 of human hepatocytes by intrasplenic injection. Mouse serum levels of human albumin were measured and used as a marker for the hepatic replacement ratio by human hepatocytes (Hasegawa et al., 2011). The hepatic replacement ratios were calculated with respect to an in-house calibration curve, and those mice with a ratio greater than 77% were used. TK-NOG mice whose serum ALT levels had not been elevated sufficiently by the GCV treatments were used as control TK-NOG mice. Administration of drugs to and sample collections from the mice were performed in compliance with protocols approved by the Sekisui Medical Animal Care and Use Committee.
Control TK-NOG and humanized chimeric mice received a single oral administration of diclofenac sodium (10 mg/kg as free form) or synthetic metabolite II (10 mg/kg), and blood samples were collected for analyses at 15 and 30 minutes and 1, 4, 8, 24, and 48 hours. Three weeks later, a group of the same humanized chimeric mice were administered the same dose of diclofenac sodium orally once per day for 14 days, and blood samples were taken at 8 and 24 hours after each dose. Food and water were given ad libitum during the drug treatment. For the biliary excretion study, a control TK-NOG mouse and a humanized chimeric mouse, in which a polyethylene tube was cannulated into the bile duct, received diclofenac sodium (10 mg/kg as free form) or synthetic metabolite II (10 mg/kg), and bile samples were collected for 0–24 hours on dry ice.
Analyses of Plasma Samples.
Diclofenac, II, IV, and VI were quantified by liquid chromatography–tandem mass spectrometry (LC-MS/MS). To a 15- or 30-μl aliquot of control plasma, the same volume of a standard working solution of diclofenac sodium or synthetic metabolite II was added to prepare the standard sample. To a 15- or 30-μl aliquot of the sample plasma, the same volume of 50% (v/v) methanol in water was added to prepare the analytical sample. To the analytical and standard samples, 100 μl of internal standard solution (25 nM) was added. The tube was closed and mixed vigorously for ca. 10 seconds using vortex mixing and subsequently centrifuged (15,000g, 4°C, 10 minutes). The supernatant was pipetted into an autosampler vial, and 50 μl of the sample was injected into the column. The LC-MS/MS analysis was performed using the LC20AD high-performance liquid chromatography (HPLC) system (Shimadzu, Kyoto, Japan) coupled with an API-4000 tandem mass spectrometer (AB Sciex, Foster City, CA). The column (CAPCELL PAK MG C18, 250 × 4.6 mm i.d., 5 μm; Shiseido, Tokyo, Japan) was maintained at 40°C, and diclofenac and its metabolites were eluted with a linear gradient (flow rate of 1.0 ml/min) that escalated from 15% B to 35% B by 15 minutes, was maintained at 35% B for 15 minutes, then escalated again to 90% B over 5 minutes, and was maintained at 90% B for 5 minutes [10 mM ammonium formate in distilled water (pH 5.0) (A) or acetonitrile (B)]. Analytes were detected in the positive ion mode with electrospray ionization (collision energy of 50, 30, 30, and 45 eV for diclofenac, II, IV, and VI, respectively) using selected reaction monitoring: diclofenac, [M+H]+ m/z 296 → 214; II, [M+H]+ m/z 312 → 231; IV, [M+H]+ m/z 312 → 231; VI, [M+H]+ m/z 342 → 260. The retention times of diclofenac, II, IV, VI, and 4′-hydroxydiclofenac-13C6 were 36, 25, 23, 25, and 25 minutes, respectively. The limits of quantification were 1 and 3 ng/ml in plasma, and determination coefficients (R) were >0.993 and >983 for diclofenac and II, respectively. Plasma concentrations of IV and VI were expressed as peak response since their standard reference compounds were not available.
Analyses of Bile Samples.
Diclofenac glutathione conjugates were analyzed by a liquid chromatography-multiple-stage mass spectrometer using the LC20AD HPLC system coupled with an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA). A 10- to 25-μl aliquot of bile sample was injected into the column (CAPCELL Core ADME, 150 × 2.1 mm i.d., 2.7 μm; Shiseido) without any pretreatment. The column was maintained at 40°C, and chromatography was performed with a linear gradient from 10% B to 30% B by 30 minutes at a flow rate of 0.2 ml/min [100 mM ammonium formate in distilled water (pH 5.0) (A) or acetonitrile (B)]. After the analysis, the column was washed by increasing the composition from 30% B to 100% B, and it was maintained at 100% B for 10 minutes and returned to 10% B. The electrospray ionization ion source was operated in positive ion mode, and instrumental parameters were set as follows: spray voltage 4.5 kV, capillary temperature 330°C, capillary voltage 3.0 V. Accurate mass chromatograms were obtained with a resolution of 30,000 by monitoring: M1, M2, and M3, [M+H]+ m/z 617.08394–617.09012; M4 or M5, [M+H]+ m/z 583.12308–583.12892; M6, [M+H]+ m/z 567.12825–567.13393. The calculated high-resolution mass values as protonated ions for C24H27Cl2N4O9S+ (M1, M2, and M3), C24H28ClN4O9S+ (M4 or M5), and C24H28ClN4O8S+ (M6) were m/z 617.08703, m/z 583.12600, and m/z 567.13109, respectively.
Pharmacokinetic Data Analysis for VI.
Metabolite VI was generated from diclofenac via more than two sequential metabolic reactions, and was considered to exhibit complicated pharmacokinetics. However, for a simplified simulation in the multiple-dose study, a one-compartment open model was arbitrarily applied to the plasma concentration of VI after a single oral administration of diclofenac. Assuming that both formation and elimination rates of VI follow first-order pharmacokinetics, pharmacokinetic parameters A, kel (elimination rate constant), and kform (formation rate constant), defined by the following equation, were obtained: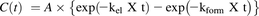 where C(t) is plasma concentration of VI at time t.
where C(t) is plasma concentration of VI at time t.
Results
Unchanged Drug and Its Metabolites after a Single Oral Administration of Diclofenac.
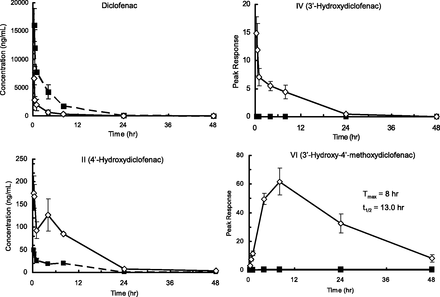
After oral administration of diclofenac sodium to control TK-NOG mice, plasma concentration of the unchanged drug peaked at 0.25 hour and declined with a t1/2 of 3.5 hours (Fig. 1). Metabolite II also reached peak concentration at 0.25 hour. However, plasma level at this time point was approximately 1/300 of the unchanged form. Although quantitative analyses could not be done for IV and VI since authentic standards were not available (expressed as peak response), we could not detect IV, and only a trace amount of VI was found in the control mouse plasma.
Plasma concentration of diclofenac and its metabolites after oral administration of diclofenac sodium (10 mg/kg as free form) to TK-NOG chimeric mice with humanized livers (―□―) and control TK-NOG mice (- -■- -). Mean ± S.D. of three experiments.
The same dose of diclofenac sodium was given to TK-NOG chimeric mice with humanized livers. Plasma levels of the unchanged drug were 2–4 times lower than those in the control mice, with a relatively shorter t1/2 of 2.4 hours (Fig. 1). Oral clearance of diclofenac in chimeric mice was 900 ml/h per kg, whereas in control TK-NOG mice it was 180 ml/h per kg, suggesting higher hepatic clearance in the humanized animal model for this drug. On the other hand, plasma concentrations of II were 3–4 times higher than those in the control mice and declined with a t1/2 of 5.6 hours. Humanized chimeric mice also had IV in their plasma which declined with a t1/2 of 4.8 hours. With gradual decrease of II and IV, metabolite VI peaked at 8 hours and declined with an apparent t1/2 of 13.0 hours, which allowed for detection of this metabolite even at 48 hours after dosing. Pharmacokinetic parameters obtained by curve fitting [A = 163 (peak response), kform = 0.19 hour−1, kel = 0.064 hour−1] were used for the multiple-dose simulation described later.
Plasma Profiles of II and VI after Oral Administration of II.
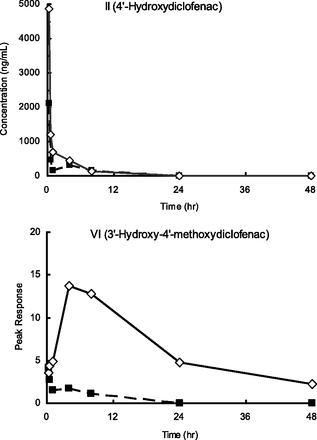
To discern the metabolic pathways of formation for the human-specific metabolite VI, 10 mg/kg synthetic metabolite II was orally administered to control TK-NOG and humanized chimeric mice. The plasma concentration of unchanged II peaked at 0.25 hour and declined rapidly in both animals (Fig. 2). The metabolic product, VI, in the humanized chimeric mouse exhibited a plasma profile similar to that obtained after administration of diclofenac. Unexpectedly, we could also detect the plasma profile of VI in the control TK-NOG mouse. However, the levels of VI were relatively low, and the area under the curve (AUC) value was 7% of that in humanized chimeric mice.
Plasma concentration of 4′-hydroxydiclofenac and 3′-hydroxy-4′-methoxydiclofenac after oral administration of 10 mg/kg 4′-hydroxydiclofenac to a TK-NOG chimeric mouse with humanized liver (―□―) and a control TK-NOG mouse (- -■- -).
Plasma Levels of VI after Repeated Administrations of Diclofenac.
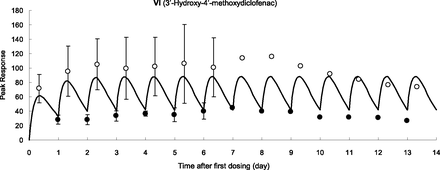
Diclofenac sodium was given orally to three TK-NOG chimeric mice with humanized livers once per day, and peak and trough levels were plotted with a simulated concentration-time curve assuming that the same pharmacokinetic conditions and compartment model for the single-dose experiment could be applied (Fig. 3). Plasma levels of VI at 8 and 24 hours gradually increased and reached plateau within 3 days. On the seventh day, one animal died and the average values of the remaining two animals tended to decrease after the ninth day. However, data did not greatly diverge from the simulation curve. Although the reason for the death was not clear, hepatic toxicity (Bort et al., 1999; Thanagari et al., 2012) or induction of intestinal injury (Zhu and Zhang, 2012) by diclofenac (LD50 of 95 mg/kg in mice; Thanagari et al., 2012) could be responsible in part for this phenomenon.
Plasma concentration of 3′-hydroxy-4′-methoxydiclofenac at 0 (●) and 8 hours (○) after repeated oral administration of diclofenac sodium (10 mg/kg as free form) to TK-NOG chimeric mice with humanized livers. Each point represents the mean ± S.D. of three (first to seventh day) or mean of two (eighth to fourteenth day) experiments.
Biliary Glutathione Conjugates with MH+ Ion of m/z 617 (M1, M2, and M3).
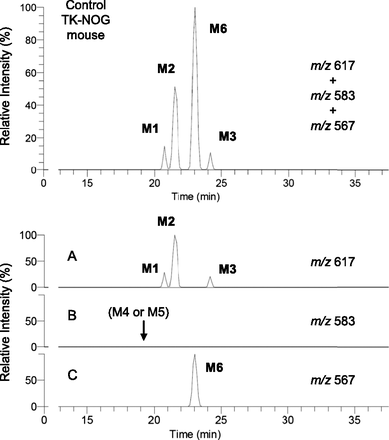
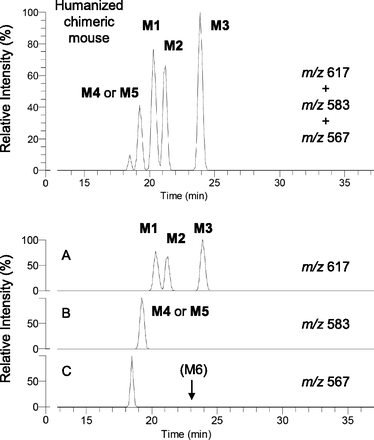
Three glutathione-conjugated metabolites of diclofenac have reportedly been detected in the bile of rats by LC-MS/MS with constant neutral loss scanning that loses 129 Da (pyroglutamate) on collision-induced dissociation (CID) from the MH+ ion of m/z 617 (Tang et al., 1999). In the present study, we monitored with integrated accurate mass detection to detect these glutathione conjugates specifically (m/z 617.08394–617.09012) and found that no interfering peaks were observed in blank bile samples (data not shown). In the bile of control TK-NOG mice treated with diclofenac sodium, three peaks were observed with retention times of 20.7, 21.5, and 24.9 minutes under the HPLC conditions described in Materials and Methods. Mass spectra of the three HPLC peaks with a CID of m/z 617 gave the same product ions at m/z 542 ([MH-75]+, loss of glycine), m/z 488 ([MH-129]+, loss of pyroglutamate), and m/z 342 ([MH-275]+, loss of the thioether moiety of glutathione), which agreed with those reported by Tang et al. (1999). Based on the similarity of the HPLC conditions and the pattern of chromatograms obtained compared to those of Tang et al. (1999), we considered that the respective peaks corresponded to M1, M2, and M3 (Fig. 4A). After oral administration of diclofenac to humanized chimeric mice, a similar chromatogram with three peaks was obtained (Fig. 5A).
Glutathione conjugate metabolites in the bile by integrated high-resolution accurate mass detection after oral administration of diclofenac sodium (10 mg/kg as free form) to a control TK-NOG mouse. Each metabolite was monitored at a corresponding mass number (A–C), and a mixed chromatogram is shown in the upper panel.
Glutathione conjugate metabolites in the bile by integrated high-resolution accurate mass detection after oral administration of diclofenac sodium (10 mg/kg as free form) to a humanized chimeric mouse. Each metabolite was monitored at a corresponding mass number (A–C), and a mixed chromatogram is shown in the upper panel.
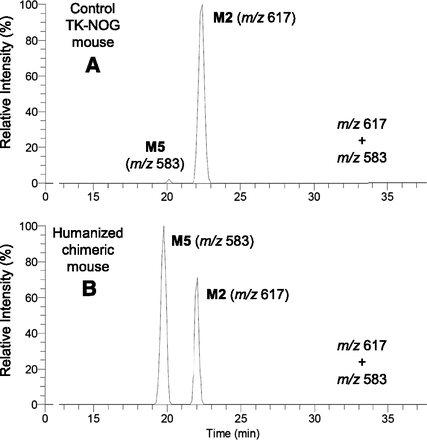
Following oral administration of synthetic metabolite II to control TK-NOG and humanized chimeric mice, a single peak at a retention time of 22 minutes was observed in the bile samples from both animals (Fig. 6, m/z 617). When the sample from the humanized chimeric mouse (Fig. 6B) was mixed with the bile obtained from the humanized mouse treated with diclofenac (Fig. 5A) and then injected into the HPLC column, the single peak of the former sample at m/z 617 coeluted with the second peak of the latter sample. Therefore, this single peak was considered as M2, as the 5-hydroxy analogs, M1 and M3, could simply not be generated from II.
Glutathione conjugate metabolites in the bile by integrated high-resolution accurate mass detection after oral administration of 10 mg/kg 4′-hydroxydiclofenac to control TK-NOG (A) and humanized chimeric mice (B).
Biliary Glutathione Conjugate with MH+ Ion of m/z 583 (M4 or M5).
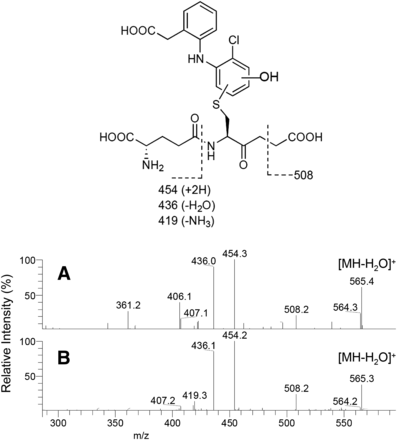
Two in vitro metabolites with MH+ ions of m/z 583 have been separately reported as human-specific glutathione conjugate metabolites of diclofenac. M4 was previously generated by incubation of diclofenac with human liver microsomes or recombinant CYP2C9 fortified with glutathione (Yan et al., 2005). The chemical structure of M4 was proposed as a 2′-hydroxy-3′-glutathionyl analog based on the assumption that this monochlorinated metabolite was generated via diclofenac-2′,3′-oxide, because M4 had not been detected in the incubation mixture of II and NADPH-fortified human liver microsomes. On the other hand, 4′-hydroxy- 2′-(glutathion-S-yl)monoclofenac was proposed by Yu et al. (2005), and designated as M5 later (Boerma et al., 2014). Similar to M4, M5 was generated in an incubation mixture of diclofenac and human liver microsomes fortified with glutathione, and its chemical structural assignment was obtained via comparison of mass and 1H NMR spectra with those of a chemically synthesized authentic standard. In the present study, no monitored peaks were found in the bile of the control TK-NOG mouse dosed with diclofenac at m/z 583 (m/z 583.12308–583.12892) (Fig. 4B). However, bile from the humanized chimeric mouse gave a single peak presumably produced by the transplanted human hepatocytes (Fig. 5B). When the ion at m/z 583 was subjected to CID (Fig. 7A), the product ions at m/z 508 ([MH-75]+), m/z 454 ([MH-129]+), and m/z 436 ([MH-147]+) were obtained as separately reported by Yan et al. (2005) for M4 and Yu et al. (2005) for M5. To determine that this biliary metabolite is M4 or M5, further studies including isolation of the metabolite from the bile sample and subsequent NMR analyses are inevitable.
Collision-induced dissociation spectra of product ion m/z 583 of the human-specific metabolite (M4 or M5) in the bile of humanized chimeric mice treated with diclofenac sodium (A) and 4′-hydroxydiclofenac (B).
Oral administration of synthetic metabolite II to a humanized chimeric mouse also gave a single peak at m/z 583 (Fig. 6B), and CID produced the same product ions as those after diclofenac treatment (Fig. 7B). This conjugate was considered to be M5, based on the chemical structure of II, and coeluted with the corresponding peak of the bile sample from chimeric mice dosed with diclofenac (Fig. 5B). However, M4 and M5 are positional isomers, and possible coincidence in the HPLC retention times cannot be ruled out. Although M5 (and M4) had been considered human specific, the same peak was unexpectedly found in the bile sample from a control TK-NOG mouse dosed with II (Fig. 6A). However, it was much smaller than that in the humanized chimeric mouse, and was not observed in the control mouse when dosed with diclofenac (Fig. 4B).
Biliary Glutathione Conjugate with MH+ Ion of m/z 567 (M6).
2′-(Glutathion-S-yl)monoclofenac (we designated this conjugate “M6”) was found first in incubation mixtures of diclofenac with human liver microsomes (Wen et al., 2008). In vitro studies using various recombinant human P450s fortified with glutathione and human glutathione-S-transferase P1-1 demonstrated that all of the 14 tested P450 isozymes were capable of forming M6 (Boerma et al., 2014). Furthermore, this reaction was NADPH-independent with M6 produced to some extent by flavin-containing monooxygenase or even horseradish peroxidase, suggesting the involvement of one-electron oxidation. In the present study, a large single peak at m/z 567 (m/z 567.12825–567.13393) was obtained in the bile sample from a control TK-NOG mouse (Fig. 4C). This metabolite gave, on a CID of m/z 567, product ions at m/z 492 ([MH-75]+) and m/z 438 ([MH-129]+), as reported by Boerma et al. (2012). Moreover, this single peak at m/z 567 coeluted on HPLC with an in vitro reference product of M6 provided by Jan N. M. Commandeur of VU University (see Materials and Methods). Although this metabolite was reportedly generated in in vitro studies using various human specimens, the peak of M6 in the bile of a humanized chimeric mouse was not found (Fig. 5C).
Discussion
Potential pathways of diclofenac metabolism are illustrated in Fig. 8. Hydroxylation at the 4′-position is one of the major metabolic routes of diclofenac, and metabolite II has been used as a marker of human CYP2C9 activity in humanized animal models. The in vitro assessment using liver microsome preparations from humanized uPA/severe combined immunodeficient chimeric mice with varying replacement ratios showed that the formation of II correlated well with hepatic replacement ratio, relative expression levels of CYP2C9 mRNA, and CYP2C9 protein content (Katoh et al., 2004; Tateno et al., 2004). Recently, Hu et al. (2013) produced humanized TK-NOG chimeric mice using hepatocytes from donors with different genetic backgrounds. After oral dosing of diclofenac to this chimeric mouse model, plasma levels of II were extremely low in CYP2C9 *2/*3, moderate in CYP2C9 w/*2, and highest in CYP2C9 w/w genotypes. More recently, Bateman et al. (2014) introduced diclofenac as a suitable substrate for the in vivo evaluation of humanized uPA/severe combined immunodeficient chimeric mice with monitoring of II reaction product. In the present study, human hepatocytes exhibiting significant CYP2C9 metabolic activity were transplanted into TK-NOG mice, and higher plasma concentrations of II were observed in the humanized chimeric mice relative to control mice. Hydroxylation at the 4′-position is also one of the major routes of metabolism in mice (Sarda et al., 2012). However, it is possible that subsequent conjugation of II to its taurine, glucuronic acid, or glucose conjugates might have resulted in lower plasma concentrations of this metabolite in the control mice.
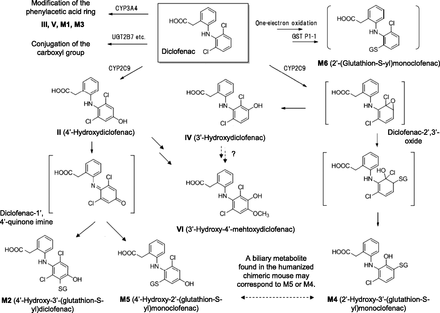
Proposed metabolic pathways of diclofenac in humans deduced from the metabolic studies with TK-NOG chimeric mice with humanized livers. VI was confirmed to be generated from II, but formation of VI from IV was not clear since the synthetic version of IV was not available. The biliary metabolite with the parent ion of m/z 583 may correspond to M4 (Yan et al., 2005) or M5 (Yu et al., 2005) proposed in separate studies using human liver microsomes. III, 5-hydroxydiclofenac; V, 4′,5-dihydroxydiclofenac.
On the other hand, hydroxylation of diclofenac at the 3′-position has been reported only with nonhuman primates or humans (Stierlin et al., 1979; Stierlin and Faigle, 1979). After oral dosing of diclofenac to humanized chimeric mice, significant levels of IV were observed, whereas this metabolite was completely missing in the plasma of control mice, suggesting generation of IV by the transplanted human hepatocytes. Human-specific VI is a complex metabolite which may need both 3′- and 4′-hydroxylation followed by methylation at the 4′-position of 3′,4′-dihydroxydiclofenac. In humanized chimeric mice, levels of VI in plasma reached a maximum at 8 hours and then declined with a t1/2 of 13.0 hours. These parameters seem to be comparable to the respective values of 12 and 80 hours in humans (Faigle et al., 1988), and could be used to retrospectively predict the distinct pharmacokinetic behavior of VI in clinical practice. It had previously not been known whether VI was generated from diclofenac via II or IV as an intermediate metabolite. We then administered synthetic metabolite II to humanized chimeric mice and observed a plasma profile of VI similar to that after diclofenac dosing. Possible formation of VI from IV remains unknown since synthetic IV was not available.
Degen et al. (1988) reported that, following multiple twice-daily dosing of a retard formulation of diclofenac to patients, trough plasma concentrations of VI were about 6 times larger than the unchanged drug. In the present study, diclofenac was given once per day to humanized chimeric mice, with plasma peak and trough concentrations of VI reaching a plateau within 3 days of treatment, and was retained at this level for a week. These data were not greatly different from the simulated plasma profile based on a once-per-day regimen. More frequent dosing or administration of a delayed-release formulation may, therefore, result in more significant accumulation of VI.
Metabolite VI showed about 300 times less activity than the parent compound in inhibiting prostaglandin synthesis in vitro, and the anti-inflammatory effects in vivo were very weak (Faigle et al., 1988). According to the Metabolites in Safety Testing guidance by the Food and Drug Administration and the subsequent modified guidance by the International Conference on Harmonization [ICH M3(R2); Food and Drug Administration, 2010], safety of human-specific metabolites occurring at exposure greater than 10% of total drug-related exposure should be evaluated before entering large-scale clinical trials. Metabolite VI was one such human-specific metabolite, and since it was generated through more than one metabolic reaction, it could not be predicted by conventional in vitro systems such as human hepatocytes, hepatic S-9 fraction, and microsomes (Dalvie et al., 2009). When AUC0-∞ values for each metabolite reported by Degen et al. (1988) were included in the calculation, VI comprised 83% of total drug-related exposure (diclofenac plus metabolites II–VI) since elimination of VI was slow. This metabolite was largely pharmacologically inactive and deemed not to be a safety concern. However, if a human-specific metabolite such as VI is found in a later stage of new drug development and exhibits unexpected pharmacokinetic behavior, development of the new drug could be delayed. To address this concern, chimeric mice with humanized livers are very useful for the prediction of circulating human metabolites before entering first-in-human trials (Kamimura et al., 2010).
Toxicity of diclofenac to hepatocytes is reduced in vitro by the addition of cytochrome P450 inhibitors (Bort et al., 1999), and that to the small intestine is ameliorated in intestinal epithelium–specific P450 reductase knockout mice (Zhu and Zhang, 2012), suggesting involvement of P450-mediated bioactivation. As evidence of bioactivation, three glutathione-trapped metabolites, M1, M2, and M3, were identified in the bile of rats (Tang et al., 1999). Among these metabolites, M1 and M3 were generated by oxidation of the phenyl acetic acid moiety followed by glutathione conjugation, whereas formation of M2 was initiated by oxidation of the dichlorophenyl ring (Fig. 8). In the present study, similar extracted ion chromatograms were obtained from the bile of the control TK-NOG mice, indicating the same reactions also occur in mouse species. In addition, M6, reported to be generated in vitro by one-electron oxidation of diclofenac (Boerma et al., 2014), was also detected in mouse bile. In metabolism studies using 3H-labeled diclofenac, Sarda et al. (2012) reported the major metabolites in mouse feces were taurine conjugates of II and diclofenac, whereas Bateman et al. (2014) reported the major metabolite in mouse bile was diclofenac acyl glucuronide. Although their results did not agree well, both reports denied or did not mention formation of glutathione conjugate metabolites of diclofenac. However, the present study suggested that glutathione-trapped metabolites of diclofenac were generated to some extent even in mice. Metabolites M1, M2, and M3 were also detected in the bile of humanized chimeric mice, which suggested these glutathione-conjugate metabolites were possibly generated at least in part by transplanted human hepatocytes. Involvement of the human hepatocytes in the formation of M6, an in vitro human metabolite, could not be demonstrated.
Monochlorinated metabolites M4 and M5 have been previously detected in incubation mixtures of diclofenac with human liver microsomes fortified with glutathione (Yan et al., 2005; Yu et al., 2005). Following oral administration of diclofenac to a control TK-NOG mouse, no glutathione conjugates at m/z 583 were detected in the bile. On the other hand, a single peak producing a mass spectrum identical to those of M4 and M5 was observed in the humanized chimeric mouse. This result was puzzling, since it was uncertain if this in vivo metabolite corresponds to M4 or M5. If it is assumed that this human-specific metabolite is M4, this metabolite was generated via diclofenac-2′,3′-oxide as proposed by Yan et al. (2005) (Fig. 8). This hypothesis is consistent with our finding that another possible product from the arene oxide, IV, was completely missing in the plasma of control mice and considered to be human-specific. Bioactivation of diclofenac to diclofenac-2′,3′-oxide was mediated by human CYP2C9 (Yan et al., 2005), and mice may lack this metabolic pathway. As supporting evidence in the report by Yan et al. (2005), incubation of II with human liver microsomes did not produce M4. However, this result was inconsistent with our finding that a peak at m/z 583 was also detected after administration of II to the humanized chimeric mouse. If it is assumed that the human-specific metabolite is M5, detection of the peak in the humanized chimeric mouse treated with II provides a rational basis for the proposed diclofenac-1′,4′-quinone imine intermediate for the formation of this metabolite (Yu et al., 2005) (Fig. 8). In this scenario, formation of M5 from the quinone imine intermediate was either exclusively or predominantly a human metabolic reaction. Even a control TK-NOG mouse dosed with 10 mg/kg II gave a relatively small peak at m/z 583, probably because exposure of the mouse to II was much higher than that after administration of the same dose of diclofenac. However, further studies including NMR analysis of the biliary metabolite to distinguish the potential positional isomers are necessary.
In conclusion, we could mimic the distinct plasma concentration profile of the human-specific metabolite of diclofenac (VI) by TK-NOG mice with humanized livers. Glutathione conjugate metabolites were also detected in the bile samples, providing a new means of studying glutathione-dependent inactivation of reactive human metabolites in vivo. As with the conventional humanized animal models, humanized TK-NOG chimeric mice were considered to be a useful tool for metabolism studies in new drug development.
Acknowledgments
The authors thank Jan N. M. Commandeur (VU University Amsterdam) for providing the in vitro reference material for M6; Daisuke Morita (Seikagaku Corporation, Tokyo, Japan) for providing HPLC conditions for the separation of diclofenac and its metabolites II, IV, and VI; and Faraz Kazmi (XenoTech,) for correcting the English manuscript.
Authorship Contributions
Participated in research design: Kamimura.
Conducted experiments: Ito, Nozawa, Nakamura, Chijiwa.
Contributed new reagents or analytic tools: Kuronuma, Ohnishi, Suemizu.
Performed data analysis: Nagatsuka.
Wrote or contributed to the writing of the manuscript: Kamimura, Ito, Ninomiya.
Footnotes
- Received October 20, 2014.
- Accepted December 10, 2014.
Abbreviations
- II
- 4′-hydroxydiclofenac
- IV
- 3′-hydroxydiclofenac
- VI
- 3′-hydroxy-4′-methoxydiclofenac
- ALT
- alanine aminotransferase
- CID
- collision-induced dissociation
- GCV
- ganciclovir
- HPLC
- high-performance liquid chromatography
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- M1
- 5-hydroxy-4-(glutathion-S-yl)diclofenac
- M2
- 4′-hydroxy-3′-(glutathion-S-yl)diclofenac
- M3
- 5-hydroxy-6-(glutathion-S-yl)diclofenac
- M4
- 2′-hydroxy-3′-(glutathion-S-yl)monoclofenac
- M5
- 4′-hydroxy-2′-(glutathion-S-yl)monoclofenac
- M6
- 2′-(glutathion-S-yl)monoclofenac
- P450
- cytochrome P450
- t1/2
- half-life
- uPA
- urokinase-type plasminogen activator
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics