Abstract
Propranolol is a nonselective β-adrenergic blocker used as a racemic mixture in the treatment of hypertension, cardiac arrhythmias, and angina pectoris. For study of the stereoselective glucuronidation of this drug, the two propranolol glucuronide diastereomers were biosynthesized, purified, and characterized. A screen of 15 recombinant human UDP-glucuronosyltransferases (UGTs) indicated that only a few isoforms catalyze propranolol glucuronidation. Analysis of UGT2B4 and UGT2B7 revealed no significant stereoselectivity, but these two enzymes differed in glucuronidation kinetics. The glucuronidation kinetics of R-propranolol by UGT2B4 exhibited a sigmoid curve, whereas the glucuronidation of the same substrate by UGT2B7 was inhibited by substrate concentrations above 1 mM. Among the UGTs of subfamily 1A, UGT1A9 and UGT1A10 displayed high and, surprisingly, opposite stereoselectivity in the glucuronidation of propranolol enantiomers. UGT1A9 glucuronidated S-propranolol much faster than R-propranolol, whereas UGT1A10 exhibited the opposite enantiomer preference. Nonetheless, the Km values for the two enantiomers, both for UGT1A9 and for UGT1A10, were in the same range, suggesting similar affinities for the two enantiomers. Unlike UGT1A9, the expression of UGT1A10 is extrahepatic. Hence, the reverse stereoselectivity of these two UGTs may signify specific differences in the glucuronidation of propranolol enantiomers between intestine and liver microsomes. Subsequent experiments confirmed this hypothesis: human liver microsomes glucuronidated S-propranolol faster than R-propranolol, whereas human intestine microsomes glucuronidated S-propranolol faster. These findings suggest a contribution of intestinal UGTs to drug metabolism, at least for UGT1A10 substrates.
Propranolol, [1-isopropylamino-3-(1-naphtoxy)-2-propanol], is a nonselective β-adrenergic blocking agent used as a racemic mixture in the treatment of hypertension, cardiac arrhythmias, and angina pectoris. Because the interactions of drugs with drug-metabolizing enzymes are often stereoselective, it was of considerable interest to investigate the interactions of chiral entities, here propranolol enantiomers, with proteins that bind and metabolize them, here the UDP-glucuronosyltransferases (UGTs; EC 2.4.1.17).
Propranolol is metabolized through various pathways, including conjugation, side-chain oxidation, and ring oxidation. It is glucuronidated into two diastereomeric O-glucuronides in human, as well as in dog and rat (Thompson et al., 1981; Bai and Walle, 1984). Glucuronidation involves the transfer of the glucuronic acid moiety of UDP-glucuronic acid (UDP-GA) to endogenous or exogenous substrate molecules containing a suitable group, usually a hydroxyl, amine, or carboxyl group (Tukey and Strassburg, 2000). The glucuronides formed are more hydrophilic than the parent aglycones, so that they are more readily excreted from the body through the urine or bile. Glucuronidation is catalyzed by the UGTs, a family of membrane-bound enzymes of the endoplasmic reticulum (Radominska-Pandya et al., 1999; Tukey and Strassburg, 2000; Ouzzine et al., 2003; Wells et al., 2004).
The human genome contains some 19 active UGT-encoding genes, which are divided into three subfamilies: 1A, 2A, and 2B (Mackenzie et al., 2005). The UGT1A isoforms are encoded by a single large gene on chromosome 2, and the processing of mature mRNA for individual UGT1As is governed by exon sharing (Ritter et al., 1992; Mackenzie et al., 1997, 2005). The result of this exon sharing is that the amino acid sequence of the C-terminal half of all the UGT1As is identical. The primary structure of the N-terminal half, the N-terminal domain, of the UGT1As is also fairly well conserved, particularly among UGTs 1A7–1A10 (about 82% identity). This high homology is probably reflected in the activity of these four UGTs toward entacapone (Luukkanen et al., 2005), an aglycone that was previously assumed to be highly specific for UGT1A9 (Lautala et al., 2000). In contrast, our recent study on dobutamine glucuronidation revealed that, whereas UGTs 1A7–1A9 primarily glucuronidated this drug at the catecholic meta hydroxyl, UGT1A10 additionally produced catecholic para glucuronide of dobutamine (Alonen et al., 2005). Hence, since UGT1A9 was previously implicated in the glucuronidation of propranolol (Li et al., 2001), it was of interest to test the activity of UGT1A10 toward this drug.
About 17% of the propranolol dose is metabolized in human through stereoselective glucuronidation (Silber et al., 1982; Walle et al., 1985). When racemic propranolol was administered, the concentration of S-propranolol glucuronide (S-pg) was higher than that of R-propranolol glucuronide (R-pg) in both plasma and urine (Silber et al., 1982; Luan et al., 2005). Two UGTs, 1A9 and 2B7, were earlier found to catalyze propranolol glucuronidation (Coffman et al., 1998; Li et al., 2001). UGT2B7 is probably the only human UGT for which its stereoselectivity in propranolol glucuronidation has been tested and revealed no significant preference for R-or S-propranolol (Coffman et al., 1998). It was relevant, therefore, to find out which human UGT isoform(s) is involved in stereoselective propranolol glucuronidation.
We have examined the glucuronidation of R- and S-propranolol by human UGTs. Interesting differences were revealed in the stereoselectivity of individual recombinant UGTs, as well as between microsomes from human liver and intestine.
Materials and Methods
Materials.RS-Propranolol-HCl, R(+)- and S(–)-propranolol-HCl, UDP-GA, and d-saccharic acid 1,4-lactone were purchased from Sigma Chemicals (St. Louis, MO). Solvents were of HPLC grade. All other chemicals were of analytical reagent grade. Human liver and intestinal microsomes were purchased from Gentest (Woburn, MA).
Microsomes of liver homogenates of male Sprague-Dawley rats treated with Aroclor 1254, a mixture of polychlorinated biphenyls, were used for synthesis of glucuronide reference standards. Rat liver microsomes were prepared as described previously (Luukkanen et al., 1997). The treatment of the animals was approved by the local Ethical Committee for Animal Studies. Recombinant human UGTs were expressed in baculovirus-infected insect cells as described previously (Kurkela et al., 2003; Kuuranne et al., 2003; Finel et al., 2005; M. Kurkela, A.-S. Patana, P. I. Mackenzie, J. Hirvonen, A. Goldman, and M. Finel, manuscript in preparation). Protein concentrations were determined by the BCA method (Pierce Biotechnology Inc., Rockford, IL). The relative expression level of each recombinant UGT was determined by dot-blot analyses using anti-His-tag antibodies, as detailed elsewhere (Kurkela et al., 2004).
Biosynthesis of Glucuronides. Pure glucuronides of R(+)- and S(–)-propranolol were biosynthesized and purified for the preparation of standards for the quantitative investigation of propranolol glucuronides. Larger-scale syntheses were carried out in the presence of rat liver microsomes (1 mg/ml) and 5 mM optically pure propranolol enantiomer, either R(+) or S(–), in a total volume of 35 or 37 ml, respectively. The syntheses were carried out in the presence of 50 mM Na-K-phosphate buffer (pH 7.4), 5 mM d-saccharic acid 1,4-lactone, and 5 mM MgCl2. The reactions were started by the addition of UDP-GA, to a final concentration of 5 mM. Incubations were done in an Erlenmeyer flask at 37°C (in a water bath) for 16 h and terminated by transferring the vessel to an ice bath and cooling for 20 min; this was followed by 15 min of centrifugation at 9000 rpm. The resulting precipitates were washed with buffer and centrifuged again. The supernatants were combined and the glucuronides were purified by solid-phase extraction on C18 cartridges (Isolute 500-mg MFC18), which were preconditioned by washing with methanol (5 ml) and HPLC-water (5 ml). The amount of solution per column was 5 ml and the cartridges were washed two times with 5 ml of water. The analytes were eluted three times with 5 ml of 60% methanol in water. Each eluate fraction was analyzed, and fractions containing propranolol glucuronides were combined. The methanol was evaporated and the aqueous residue was frozen and dried under vacuum with diphosphorus pentoxide until constant mass was reached. The dried glucuronides were stored at –20°C.
HPLC Analyses. The separation and quantification of propranolol glucuronides by HPLC were carried out using an Agilent model 1100 liquid chromatograph with fluorescence detection (Hewlett-Packard, Waldbronn, Germany). The Chirobiotic T column (250 × 4.6 mm i.d., 5-μm particle size; Chirobiotic, Whippany, NJ) with a precolumn of the same material (Chirobiotic T 20 × 4.0 mm) was used under ionic organic mode conditions. The mobile phase was methanol/acetic acid/triethyl amine (100:0.2:0.1), which was filtered through a 0.45-μm filter and used at a flow rate of 1.5 ml/min. The detection wavelengths were 227 (excitation) and 336 (emission) nm, and the column temperature was 30°C. The injection volume was 50 μl. Reference incubations were carried out similarly, but in the absence of UDP-GA. The position and sequence of elution of the individual isomers were confirmed by analysis of incubation samples of optically pure enantiomers. Possible racemization and decomposition of the pure propranolol enantiomers were examined by HPLC after 18 h of incubation under the conditions described above.
Stock solutions of R- and S-propranolol glucuronides in methanol, at a concentration of 0.4 mM, were prepared and stored at 4°C. Standard calibration working solutions containing both glucuronide diastereomers were prepared in methanol at concentrations of 0.01 to 2.0 μM per glucuronide diastereomer. The samples for the calibration curves were prepared, in duplicate, by spiking 50 μl of the working solution into 50 μl of blank incubation matrix to obtain glucuronide concentrations of 0.005 to 1.0 μM. The analytical method was validated with respect to specificity, accuracy, precision, and limit of quantification as recommended by Shah et al. (2000). The limits of detection and quantification were determined at signal to noise ratios of 3 and 10, respectively. The stability of the analyte in the matrix during the sample storage period was assessed by analyzing spiked propranolol glucuronide samples immediately after preparation and again after 16 h, a typical time for the HPLC batch analysis. Intra-assay variability was determined by analyzing five parallel samples, and interassay variability by analyzing samples on five separate days.
Identification of Glucuronide Conjugates. The synthesized glucuronides were identified by LC-MS and liquid chromatography-tandem mass spectrometry using a PerkinElmer Sciex API 3000 (Sciex, Concord, ON, Canada) triple quadrupole mass spectrometer with turbo ion spray source, operated in positive-ion mode. Nitrogen was used as the curtain and collision gas and purified air as the nebulizing gas. The capillary voltage was 5000 V, declustering potential 20 V, and collision energy in the MS-MS experiment 43 V. The mass range in the LC-MS experiment was m/z 100 to 600, and the ion source temperature was 400°C. The chromatographic conditions were the same as those described above, except that the mobile phase was 0.1% ammonium acetate in methanol, used at a flow rate of 1 ml/min. The usual mobile phase was excluded because of the ion suppression of triethyl amine. The retention order of the analytes was unaffected by the changes in mobile phase composition and flow rate. The concentration of the analytes was 10 μM dissolved in mobile phase. In the tandem mass spectrometric mode, the sample was directly injected into the ion source with a Harvard syringe pump at a flow rate of 5 μl/min, and in LC-MS mode, the injection volume was 10 μl. The purity of the glucuronides was assessed by LC-UV analysis at 210 nm, a short wavelength where most molecules absorb, and by LC-fluorescence analysis.
Activity Analyses of UGTs. Activity assays were performed in the presence of 5 mM UDP-GA, 5 mM d-saccharic acid 1,4-lactone, 50 mM Na-K-phosphate buffer (pH 7.4), and 5 mM MgCl2. The concentrations of protein and substrates were selected according to the goal of the analysis. The aglycones, either racemic mixture or individual propranolol enantiomers, were added as dimethyl sulfoxide solutions so that the final dimethyl sulfoxide concentration was 2% in all assays. The reactions were started by the addition of UDP-GA and the samples were incubated at 37°C in a shaking dry bath for 30 to 60 min. This incubation time was found to be within the linear range for both the protein and substrate concentrations. The reactions were stopped by the addition of ice-cold methanol, placement of the reaction mixtures in ice for 20 min, and 5 min of centrifugation at 14,000 rpm. The supernatant was used for the analysis of glucuronides.
Screening of Recombinant UGTs. Propranolol glucuronidation activity was examined for 15 recombinant human UGTs, namely 1A1, 1A3–1A10, 2B4, 2B7, 2B10, 2B15, 2B17, and 2B28, all of which were expressed in baculovirus-infected insect cells (Kurkela et al., 2003; Kuuranne et al., 2003; Finel et al., 2005). The initial incubations were performed in the presence of 0.2 mg/ml membrane protein and racemic propranolol at 0.25, 1, and 5 mM for 60 min. Those UGTs that exhibited activity were further assayed in the presence of either 1 mM racemic propranolol or 0.5 mM pure S- or R-enantiomer. The protein concentration in these latter assays, 0.2 to 1 mg/ml, was adjusted to ensure the production of sufficient amounts of propranolol glucuronides for quantitative determination under conditions of high signal to noise ratio. Control incubations were carried out in the presence of the highest aglycone substrate concentration and in the absence of UDP-GA. The activity of human liver and intestinal microsomes was screened similarly, at a protein concentration of 0.2 mg/ml and at incubation times of 30 and 60 min, respectively. Analyses were done in triplicate or duplicate throughout the study. For comparable results among the UGTs, the relative expression of each recombinant UGT was determined by immunodetection in parallel with the other tested UGTs. The specific activity values were then divided by the relative expression values to obtain the normalized activities.
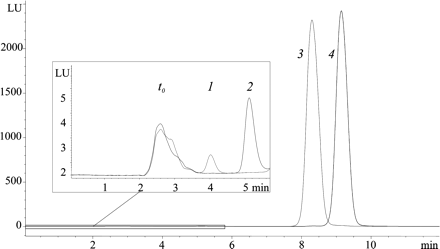
Chromatographic separation of S- and R-propranolol, as well as the corresponding glucuronides. S-Propranolol or R-propranolol, 0.1 mM, was incubated with recombinant human UGT1A10 at a protein concentration of 0.2 mg/ml for 60 min. The chromatographic conditions are detailed under Materials and Methods. The elution order and retention times were S-propranolol glucuronide (1; 4.1 min), R-propranolol glucuronide (2; 5.3 min), S-propranolol and R-propranolol (3; 8.3 and 4; 9.1 min, respectively). LU, luminescence units.
Kinetic Analyses. The specific activities of the four selected UGTs were determined at seven substrate concentrations, from 25 to 2500 μM. Kinetic constants for S- and R-propranolol for isoforms active in propranolol glucuronidation were obtained by fitting kinetic models to experimental data with no weighting, using GraphPad Prism version 4.02 for Windows (GraphPad Software Inc., San Diego, CA). The three models were: 1) the Michaelis-Menten equation,  where v is the rate of reaction, Vmax is the maximum velocity, Km is the Michaelis-Menten constant (substrate concentration at 0.5 Vmax), and S is the substrate concentration; 2) the substrate inhibition model (Cornish-Bowden, 1995; Houston and Kenworthy, 2000),
where v is the rate of reaction, Vmax is the maximum velocity, Km is the Michaelis-Menten constant (substrate concentration at 0.5 Vmax), and S is the substrate concentration; 2) the substrate inhibition model (Cornish-Bowden, 1995; Houston and Kenworthy, 2000), 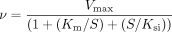 where Ksi is the constant describing the substrate inhibition interaction; and 3) the Hill equation, which describes sigmoidal kinetics,
where Ksi is the constant describing the substrate inhibition interaction; and 3) the Hill equation, which describes sigmoidal kinetics,  where S50 is the substrate concentration resulting in 50% of Vmax and n is the Hill coefficient. In some cases, none of the kinetic mechanisms gave a satisfactory fit, and where no clear preference for a certain model was observed, the simpler equation was selected. The goodness of fit of the data to the respective kinetic models was assessed from the r2 values, parameter standard error estimates, and 95% confidence intervals.
where S50 is the substrate concentration resulting in 50% of Vmax and n is the Hill coefficient. In some cases, none of the kinetic mechanisms gave a satisfactory fit, and where no clear preference for a certain model was observed, the simpler equation was selected. The goodness of fit of the data to the respective kinetic models was assessed from the r2 values, parameter standard error estimates, and 95% confidence intervals.
Results
The biosynthesis of the two propranolol glucuronides was carried out using rat liver microsomes. In preliminary studies, propranolol was found to be stable in phosphate buffer, and less than 4% of the substrate decomposed during 18 h of incubation (data not shown). The propranolol enantiomers did not racemize, and when the substrate was a purified enantiomer rather than a racemic mixture, only one glucuronide was found after 18 h of incubation. Control incubations of the microsomes in the presence of propranolol enantiomers or racemic propranolol but without UDP-GA showed only the aglycone peaks. The addition of UDP-GA gave rise to additional peaks that were eluted before the substrate peaks (Fig. 1). The identity of the new peaks as the two propranolol glucuronides was ascertained by LC-MS as described below. The elution order and retention times in our HPLC method were S-pg, 4.1 min; R-pg, 5.3 min; S-propranolol and R-propranolol, 8.3 and 9.1 min, respectively (Fig. 1).
The biosynthesis of the propranolol glucuronides was carried out in the presence of a 5 mM concentration of the respective optically pure enantiomer for 16 h. This substrate concentration was selected because the Km values of the propranolol enantiomers in rat liver microsomes were about 1.6 and 0.9 mM for the R- and S-propranolol, respectively, and substrate inhibition was observed above 5 mM propranolol (results not shown). The final yields after the purification processes were 3.34 mg (R-pg) and 4.48 mg (S-pg), values that are 4.4% and 5.6% of the theoretical yield.
The two propranolol glucuronides were characterized by LC-MS. Under the conditions of these experiments the S- and R-glucuronides were eluted at retention times of 7.1 and 8.8 min, respectively, and analyzed in the positive ion mode. The mass spectra of the glucuronides showed an abundant protonated molecule [M + H]+ at m/z 436.3 and its sodium adduct ion at m/z 458.3. No fragmentation and no propranolol traces were detected in these LC-MS analyses. In the subsequent MS-MS analysis, the precursor ion was [M + H]+m/z 436. As expected, no difference was observed between the MS-MS spectra of the two glucuronide diastereomers. The main fragment at m/z 260 was produced by the loss of the glucuronic acid. Other intense peaks originated from further fragmentation of the m/z 260 ion. On the basis of the LC analysis with UV and fluorescence detection, the R-pg and S-pg were 98.6% and 95.4% pure, respectively.
The purified propranolol glucuronides were used to calibrate the detection and quantification system. Under the conditions of our HPLC method, the standard curves were linear (r = 0.998) between 0.005 and 1 μM. The signal to noise ratio at the lower limit of quantification was 10, determined on five parallel samples. The analytes were stable in the incubation over the time period of the analysis cycle, as assessed by five parallel RS-propranolol glucuronide samples spiked into the incubation matrix. Assessment by regular guidelines (e.g., Shah et al., 2000) indicated that the analytical method was precise and accurate (Table 1). The relative standard deviation of the interday repeatability was less than 8% at all concentration levels.
Precision and accuracy of the HPLC method for the analysis of propranolol glucuronides
The accuracy was calculated as the ratio of measured concentration and nominal concentration × 100%.
The next part of this study comprised detailed analyses of the propranolol activity of 15 recombinant human UGTs, namely, 1A1, 1A3–1A10, 2B4, 2B7, 2B10, 2B15, 2B17, and 2B28. Racemic propranolol mixture was used as substrate in the initial screen, and these UGTs that glucuronidated at least one propranolol enantiomer are shown in Fig. 2. These UGTs, i.e., 1A1, 1A9, 1A10, 2B4, 2B7, and 2B17, were then incubated in the presence of a racemic mixture of propranolol and of individual pure enantiomers. The results of this comparison are presented in Table 2. It should be noted that, in these assays, to observe a difference in activity toward one propranolol enantiomer that is dependent on the presence of the other enantiomer, we made the concentration of an enantiomer the same, 0.5 mM, regardless of the presence or absence of the other enantiomer. Hence, in the incubation of racemic mixture, the total propranolol concentration was 1.0 mM. One of the interesting results of this experiment was that enzymes such as UGT1A9 that are predominantly active toward just one of the propranolol enantiomers, S-propranolol in this case, are inhibited by the presence of the other enantiomer (Table 2).
Rates of glucuronidation of propranolol enantiomers and their dependence on the presence (racemic mixture) or absence of the other enantiomer
Formation of S-propranolol glucuronide (S-pg) and R-propranolol glucuronide (R-pg) was determined by incubations in the presence of 0.5 mM concentration of the tested enantiomer (in both the presence and absence of the other enantiomer). Values are ±S.D.
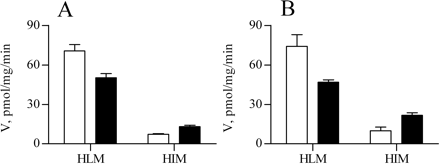
The use of individual enantiomers rather than the racemic mixture enabled quantification of the glucuronidation rates of both propranolol enantiomers, even with UGT1A1 and UGT2B17 (Table 2). The activity of the latter two UGTs was, nevertheless, very low, and they were not examined further. With the other four UGTs examined in this way, the glucuronidation rate of either enantiomer was inhibited in varying degree by the presence of the other enantiomer (Table 2). For reasons discussed below, we have also examined the propranolol glucuronidation activity of microsomes from human liver and intestine. The specific activity of the human liver microsomes (HLM) was high toward both enantiomers and, apparently, it was not affected by the presence of the other enantiomer (Table 2). This last result may indicate high capacity for propranolol glucuronidation in HLM, much higher than in human intestine microsomes (HIM; Table 2).
The turnover rates of the recombinant UGTs 1A9, 1A10, 2B4, and 2B7 in glucuronidation of propranolol enantiomers were compared in terms of their normalized activity (Fig. 2). The results of this analysis indicated that UGT1A9 is more active toward its best substrate enantiomer than UGT1A10 is toward its (Fig. 2).
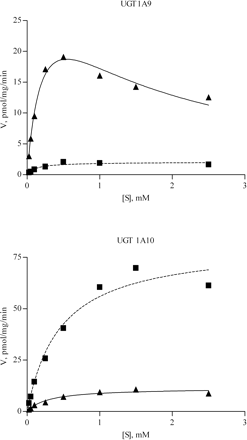
Propranolol glucuronidation by UGTs 1A9 and 1A10 largely followed Michaelis-Menten kinetics. The kinetic constants are listed in Table 3. In addition, substrate inhibition, particularly by S-propranolol with UGT1A9, was clearly seen at concentrations above 0.5 mM (Fig. 3). No substrate inhibition was observed with UGT2B4, and the reaction velocity of this UGT increased even at 2.5 mM R- or S-propranolol, the highest substrate concentration used in the other kinetic assays (Fig. 4). Two higher substrate concentrations were therefore added for the analysis of UGT2B4 to obtain a better estimate of its kinetic parameters. The glucuronidation reaction due to UGT2B4 seemed to follow different types of kinetics for the two enantiomers. The Hill equation best fitted the R-propranolol glucuronidation. The glucuronidation of S-propranolol by UGT2B4 did not produce such a sigmoidal kinetic curve except, perhaps, at very low S-propranolol concentrations (Fig. 4). UGT2B7 exhibited higher affinity than UGT2B4 for both propranolol enantiomers, but it was strongly inhibited by R-propranolol concentrations above 1.0 mM. None of the equation fit well to the data of R-propranolol glucuronidation by UGT2B7, but the S-enantiomer glucuronidation kinetics of this enzyme largely follow Michaelis-Menten kinetics (Fig. 4).
Kinetic constants (±S.E.) for S- and R-propranolol glucuronidation by recombinant human UGTs 1A9, 1A10, 2B4, and 2B7
The initial rate data were fitted to eqs. 1 to 3 and the kinetic constants were derived from the best fit. In case there was no significant difference between the tested equations, the simpler one (Michaelis-Menten) was selected.
Formation of propranolol glucuronides by recombinant human UGTs. The actual rates (A) and normalized activities (B) for S-pg (□) and R-pg (▪) are presented. The UGTs were incubated with S- or R-propranolol at an enantiomer concentration of 0.5 mM. The results are averages of three replicates ± S.D. The relative expression levels of the different recombinant UGTs that were used to calculate the normalized activity are presented in parentheses in B (see Materials and Methods). The expression level of UGT1A9, the lowest in this set, was assigned the relative value 1.0.
Quite unexpectedly, sharp differences were found in the stereoselectivity of the glucuronidation of propranolol enantiomers between UGT1A9 and UGT1A10 (Figs. 2 and 3). These assays were done using recombinant UGTs that were expressed in baculovirus-infected insect cells, and it was of interest to know whether the same enantiomer preferences are exhibited by native UGTs. There are clear differences in the expression pattern of UGT1A9 and UGT1A10, UGT1A9 being expressed in liver and UGT1A10 in intestine (Tukey and Strassburg, 2001). These differences provided an opportunity to compare recombinant and native UGTs since HLM and HIM are both commercially available. HLM and HIM were purchased and their propranolol glucuronidation activity was examined. The results (Table 2; Fig. 5) were in full agreement with the qualitative prediction; namely, the UGT1A9-containing HLM produced more S-pg and the UGT1A10-containing HIM more R-pg.
Discussion
Propranolol is a widely used drug, extensively metabolized by glucuronidation (Walle et al., 1985). Previous studies have examined propranolol glucuronidation but left much to be investigated. In particular, it was of interest to identify the individual UGTs responsible for the stereoselectivity of propranolol glucuronidation, since this would contribute to our limited understanding of the substrate specificity of the UGTs. To this end, propranolol glucuronide enantiomers were biosynthesized and a chromatographic method to separate them was developed (Fig. 1; Table 1). A large set of recombinant human UGTs was then screened for propranolol glucuronidation activity, using a racemic mixture as the substrate. The results revealed some unexpected and interesting findings (Fig. 2).
Kinetics of S-(▴) and R-propranolol (▪) glucuronidation by UGT1A9 and UGT1A10. The Michaelis-Menten, substrate inhibition, or Hill rate equations (eqs. 1–3) were fitted to the data. Each data point represents the average of at least two replicates. The kinetic constants derived from these analyses are presented in Table 3.
Propranolol glucuronidation by recombinant UGT1A9 was earlier used as a test to demonstrate the proper expression of the enzyme (Li et al., 2001). The highly pronounced stereoselectivity of UGT1A9 for S-propranolol that we have observed (Figs. 2 and 3) has not been reported previously, however. A further important finding of the present study was that UGT1A10, too, exhibits extensive stereoselectivity in propranolol glucuronidation, and, surprisingly, one that is opposite to that of UGT1A9. This reverse enantiomer preference was unexpected since the degree of primary structure identity between the human UGTs 1A9 and 1A10 is over 93% (only 32 of the 505 residues in these mature proteins are nonidentical). It is also noteworthy that UGTs 1A7 and 1A8, the other two human UGTs whose amino acid sequences are highly homologous to UGT1A9 and UGT1A10, were practically inactive in propranolol glucuronidation. These findings suggest that a few amino acid differences, perhaps even a single residue, could determine not only whether or not propranolol is a substrate for one of these four UGTs, but also which of its two enantiomers is glucuronidated at a higher rate.
Kinetics of S-(▴) and R-propranolol (▪) glucuronidation by UGTs 2B4 and 2B7. See legend to Fig. 3 for details.
The results with UGTs 1A9 and 1A10 raise the question whether the preference for the R or the S enantiomer is determined by binding affinity or by the rate of glucuronic acid transfer to the already-bound aglycone substrate. The kinetic analyses revealed that for both UGT1A9 and UGT1A10, the Km values for the two enantiomers differ much less than the corresponding Vmax values (Table 3). On this basis, it appears that the differences in affinity of these enzymes to the two propranolol enantiomers are not the major determinant in stereoselectivity.
UGT2B7 was earlier shown to catalyze propranolol glucuronidation, but no significant stereoselectivity was observed (Coffman et al., 1998). Our results (Figs. 2 and 4) are in essential agreement with that finding. Nevertheless, closer examination of our kinetic results for UGT2B7 (Table 3) reveals minor differences between the two propranolol enantiomers. These differences include substrate inhibition, which for UGT2B7 is much more pronounced in the case of R-propranolol.
The fourth human UGT that exhibited considerable propranolol glucuronidation activity was UGT2B4 (Fig. 2; Table 3). Unlike UGTs 1A9, 1A10, and 2B7, the rate of propranolol glucuronidation by UGT2B4 was neither saturated nor inhibited at the highest substrate concentrations used in this study (Fig. 4). Coupled with the relatively low affinity of UGT2B4 for either propranolol enantiomer, these results suggest that UGT2B4 might play a significant role in propranolol conjugation when the local drug concentration is very high, whereas under other conditions, its contribution is minimal. It may be noted here that the plasma concentration of propranolol in human under repeated administration at a dose level of 120 mg/day is 390 ± 113 nM (Borgstrom et al., 1981), suggesting that the glucuronidation capacity of individual UGT isoforms is not saturated.
Propranolol glucuronidation by HLM and HIM that were incubated in the presence of either racemic propranolol (A) or individual enantiomers (B). The velocity of S-pg (□) and R-pg (▪) formation ± S.D. (n = 3) is presented. The incubation conditions were 0.2 mg/ml protein, 0.5 mM per propranolol enantiomer (in both the presence and absence of the other enantiomer), and incubation times of 30 and 60 min for HLM and HIM, respectively.
Another interesting observation from the experiments with UGT2B4 was the results of the kinetic analysis, which revealed a sigmoid curve in the glucuronidation of R-propranolol but not the glucuronidation of S-propranolol (Fig. 4). The sigmoid curve may indicate positive cooperative binding. Allosteric effects could not be ruled out, however, particularly since the UGTs are oligomeric proteins. Nevertheless, a sigmoid kinetic curve might also be observed in the absence of cooperative binding due, purely, to kinetic reasons. A so-called preferred-order mechanism or the existence of multiple conformations of the enzyme could provide an explanation for the apparent cooperativity (Cornish-Bowden, 1995). In any case, it has earlier been shown that the apparent kinetic model varies both between isoforms glucuronidating the same substrate and between different substrates for the same UGT (Uchaipichat et al., 2004; Luukkanen et al., 2005).
Of the main UGTs active in propranolol glucuronidation, only UGT1A10 is not expressed in the liver, at least not at significant levels (Tukey and Strassburg, 2000). UGT1A10 is mainly expressed in extrahepatic tissues such as the bile ducts and gastrointestinal tract. UGT1A9, in turn, is not found in the small intestine (Tukey and Strassburg, 2001). It has been reported that S-pg is present in higher concentration than R-pg in human plasma and urine (Silber et al., 1982; Luan et al., 2005). The results of our study in regard to both recombinant UGTs and microsomes from human liver and small intestine are in line with the previously reported ratio of propranolol glucuronides in plasma and urine, suggesting that the main site of propranolol glucuronidation is the liver. However, it is difficult to deduce from these results the contribution of intestinal UGT1A10 to the level of R-pg.
The relatively high activity in combination with the reverse stereoselectivity of recombinant UGT1A9 and UGT1A10 (Fig. 3) suggested that there may be a qualitative difference in the glucuronidation of propranolol enantiomers between microsomes from human liver and those from human intestine. From the results of the recombinant UGTs and the known expression patterns of UGT1A9 and UGT1A10, it was predicted that HLM would produce more S-pg than R-pg, whereas HIM would yield the opposite glucuronide ratio. It was not expected, however, that the ratio of the two glucuronides would be identical with the ratios obtained using UGT1A9 and UGT1A10 (Fig. 2; Table 2), since the microsomes also contain UGT2B7 and UGT2B4, which glucuronidate both enantiomers and add to the total glucuronidation activity. The predictions about the concentration ratios of the S-pg and R-pg were tested experimentally and the results (Fig. 5) were in very good agreement with predictions, indicating a good correlation between the recombinant and native UGTs.
The role of intestinal drug glucuronidation has been thought to be of little importance because of the lower total UGT activities and UDP-GA concentrations in the intestine (Lin et al., 1999). The role of intestinal first-pass glucuronidation may be significant, however, especially if the drug is a substrate to an extrahepatically expressed UGT isoform, such as UGT1A10, and if glucuronidation is an important metabolic route for the given compound. Raloxifene may be an interesting example in this respect (Jeong et al., 2005).
Finally, in this study it was demonstrated that propranolol is mainly glucuronidated by UGTs 1A9, 1A10, 2B4, and 2B7. The results yielded new information about the substrate specificity of different human UGTs and how this may vary among closely related enzymes like UGTs 1A7–1A10. In this respect, the current findings provide new tools for further exploration of structure-function relationships among the UGTs, particularly with respect to their substrate specificity. In addition, the results for human microsomes highlight the possible contribution of intestinal UGTs for drug metabolism, particularly when the drug is a good substrate for UGT1A10 (or UGTs 1A7 or 1A8).
Acknowledgments
We are grateful to Johanna Mosorin and Sanna Sistonen for skillful technical assistance.
Footnotes
-
This study was supported by the Academy of Finland (to M.F., project 207535) and by the Emil Aaltonen Foundation (to T.S.).
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.106.010371.
-
ABBREVIATIONS: UGT, UDP-glucuronosyltransferase; HIM, human intestine microsomes; HLM, human liver microsomes; HPLC, high-performance liquid chromatography; LC-MS, liquid chromatography-mass spectrometry; UDP-GA, UDP-glucuronic acid; R-pg, R-propranolol glucuronide; S-pg, S-propranolol glucuronide.
- Received March 31, 2006.
- Accepted June 1, 2006.
- The American Society for Pharmacology and Experimental Therapeutics