Abstract
UDP-glucuronosyltransferases (UGTs) are major phase II drug metabolism enzymes that catalyze the glucuronidation of numerous endogenous and exogenous compounds. UGTs are divided into two families, UGT1 and UGT2, based on evolutionary divergence and homology. Nine UGT1A and seven UGT2B functional isoforms have been identified in humans. Glucuronidation occurs mainly in liver but also in various extrahepatic tissues, possibly affecting the pharmacokinetics. In the present study, we comprehensively determined the expression of all functional UGT1A and UGT2B isoforms in normal human tissues including liver, lung, stomach, small intestine, colon, kidney, bladder, adrenal gland, breast, ovary, uterus, and testis by semiquantitative reverse transcription-polymerase chain reaction. In addition, the expressions of these UGTs mRNA in 15 kinds of human tissue-derived cell lines were also analyzed. Many UGT isoforms were abundantly expressed in the liver, gastrointestinal tract, and kidney, supporting previous studies. Interestingly, we found that all UGTs except UGT2B17 were expressed in bladder. In steroid-related tissues, UGTs were expressed in tissue- and isoform-specific manners. Expression profiles in human tissue-derived cell lines were not necessarily consistent with those in corresponding normal tissues. Different expression profiles were observed in distinct cell lines derived from the same organ. The information presented here will be helpful for understanding the glucuronidation in various tissues and for choosing appropriate cell lines for in vitro studies.
UDP-glucuronosyltransferases (UGTs) are major phase II drug metabolism enzymes in humans (Tukey and Strassburg, 2000). UGTs catalyze the glucuronidation of numerous endogenous compounds such as bilirubin, bile acids, thyroid hormone, and steroid hormones as well as substantial exogenous substrates including therapeutic drugs, carcinogens, and environmental pollutants. Currently, 19 UGT proteins have been identified in humans, and they are divided into three subfamilies, UGT1A, UGT2A, and UGT2B, based on evolutionary divergence and homology (Mackenzie et al., 2005). The human UGT1A gene cluster located on chromosome 2q37 contains multiple unique first exons for each UGT1A and common exons 2 to 5 (Ritter et al., 1992), encoding nine kinds of the functional UGT1A subfamily. The UGT2A and UGT2B genes are located on chromosome 4q13, encoding three and seven functional proteins, respectively. The UGT2A1 and UGT2A2 are formed by differential splicing of variable first exons and common exons 2 to 6, likely the UGT1A gene. Meanwhile, UGT2A3 and each UGT2B are encoded by individual genes. Until now, the clinical significance of UGT2A protein remains to be clarified. In contrast, it is well known that UGT1A and UGT2B play important roles in the glucuronidation of a variety of endogenous and exogenous compounds.
The liver plays a central role in metabolism, including glucuronidation. Additionally, extrahepatic tissues such as the gastrointestinal tract and kidney also have a role in metabolism (Soars et al., 2002). The distribution of UGT expression in human tissues has been studied mainly in the liver and gastrointestinal tract (Strassburg et al., 2000; Tukey and Strassburg, 2000). In contrast, the expression in other extrahepatic tissues has not been fully studied.
Human tissue-derived cell lines are used as a tool for in vitro drug metabolism studies or induction studies. Hepatoma HepG2 cells and adenocarcinoma Caco-2 cells are frequently used, and the expression of selected UGT isoforms in these cell lines has been reported. However, no study determined the expression of all UGT isoforms in the cell lines. Furthermore, information concerning UGT expression in other cell lines is limited. In the present study, we comprehensively determined the expression of all human UGT isoforms in various normal tissues and human tissue-derived cell lines.
Materials and Methods
Cell Lines and Culture Condition. HepG2 (hepatocellular carcinoma), Caco-2 (colorectal adenocarcinoma), LS180 (colorectal adenocarcinoma), HEK293 (embryonic kidney), ACHN (renal cell adenocarcinoma), HK-2 (renal proximal tubule cell), SW13 (adrenocortical adenocarcinoma), H295R (adrenocortical adenocarcinoma), MDA-MB-435 (breast ductal carcinoma), and MCF-7 (breast epithelial adenocarcinoma) cells were obtained from American Type Culture Collection (Manassas, VA). HuH7 (hepatocellular carcinoma), HeLa (adenocarcinoma of the cervix of uterus), and OMC-3 (ovarian mucinous cystadenocarcinoma) cells were obtained from RIKEN BioResource Center (Ibaraki, Japan). HLE cells (hepatocellular carcinoma) were obtained from the Japan Collection of Research Biosources (Tokyo, Japan). Ishikawa (endometrial adenocarcinoma) cells were generous gifts from Dr. Masato Nishida, Tsukuba University (Ibaraki, Japan).
HLE, HuH7, SW13, MDA-MB-435, HeLa, and Ishikawa cells were cultured in DMEM (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). HepG2, Caco-2, LS180, ACHN, and MCF-7 cells were cultured in DMEM supplemented with 10% FBS (Invitrogen) and 0.1 mM nonessential amino acids (Invitrogen). HEK293 cells were cultured in DMEM supplemented with 4.5 g/liter glucose, 10 mM HEPES, and 10% FBS (Invitrogen). OMC-3 cells were cultured in Ham's F12 medium with 10% FBS (BioWhittaker, Walkersville, MD). HK-2 and H295R cells were cultured in DMEM/Ham's F12 supplemented with 10% FBS (Invitrogen), 6.7 μg/liter sodium selenite, 10 mg/liter insulin, and 5.5 mg/liter transferring (ITS-G) (Invitrogen). These cells were maintained at 37°C under an atmosphere of 5% CO2/95% air.
Total RNA from Normal Human Tissues and Cell Lines. Total RNA samples from human normal tissues were purchased from Stratagene (La Jolla, CA) (liver, colon, kidney, bladder, breast, ovary, and uterus), Clontech (Palo Alto, CA) (stomach, small intestine, adrenal gland, and testis), and Cell Applications (San Diego, CA) (lung). The liver sample was from a single donor, a 45-year-old male. The breast sample was pooled tissues from two female donors, a 39- and a 49-year-old. The colon sample was pooled tissues from two female donors, a 62- and a 67-year-old. The kidney (a 56-year-old male), lung (a 40-year-old male), ovary (a 73-year-old female), and stomach (a 50-year-old male) samples were from single donors. The bladder sample was pooled tissues from two female donors, a 24- and a 42-year-old. The uterus sample was pooled tissues from three female donors, a 54-, a 68-, and a 76-year-old. The testis sample was pooled tissues from 45 whites, ages 19 to 64. The small intestine sample was pooled tissues from 5 male/female whites, ages 20 to 61. The adrenal gland sample was pooled tissues from 62 male/female whites, ages 15 to 61. Information concerning smoking or medication of the donors was not available. Total RNA from each cell line was extracted using ISOGEN (Invitrogen).
RT-PCR Analyses. The cDNA was synthesized from total RNA using ReverTra Ace (TOYOBO, Osaka, Japan) according to the manufacturer's protocol. A 1-μl portion of the reverse-transcribed mixture was added to PCR mixtures (25 μl) consisting of 1× PCR buffer [67 mM Tris-HCl buffer (pH 8.8), 16.6 mM (NH4)2SO4, 0.45% Triton X-100, 0.02% gelatin], 1.5 mM MgCl2, 0.4 μM primers, 250 μM dNTPs, and1UofTaq DNA polymerase (Greiner Japan, Tokyo, Japan). After an initial denaturation at 94°C for 3 min, the amplification was performed by denaturation at 94°C for 30 s, annealing at an appropriate temperature for 30 s, and extension at 72°C for 30 s for 35 cycles. The final extension step was performed at 72°C for 5 min. The sequences of primers used in the present study and the annealing temperatures are shown in Table 1. The PCR products (15 μl) were analyzed by electrophoresis with 2% agarose gel and visualized by ethidium bromide staining. The specificity of all primer pairs (Supplemental Fig. 1) was confirmed by digestion of the PCR products with appropriate restriction enzymes. Expression of GAPDH mRNA was used as an internal control for the cDNA quantity and quality.
Sequences of primers and annealing temperatures used for RT-PCR analyses
Results
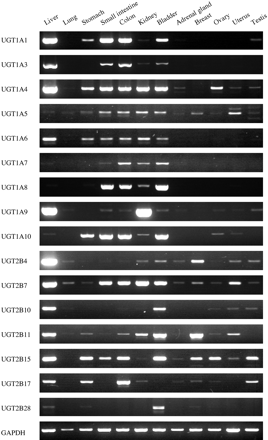
UGT mRNA Expression in Human Normal Tissues. RT-PCR analyses were performed to determine the mRNA expression of the UGT isoforms in human normal tissues (Fig. 1). UGT1A1 was highly expressed in liver, gastrointestinal tract, and bladder. UGT1A3 was expressed in a similar pattern to that of UGT1A1. Expression of UGT1A4 was widely observed in all tissues except breast. It was highest in liver and moderate in gastrointestinal tract, kidney, bladder, and ovary. UGT1A5 was expressed in gastrointestinal tract, kidney, bladder, and uterus and was marginally detected in the other tissues. UGT1A6 was expressed in liver, gastrointestinal tract, kidney, and bladder. UGT1A7 and UGT1A8 were detected in small intestine, colon, kidney, and bladder. UGT1A9 was highly expressed in liver and kidney and marginally in small intestine, colon, bladder, and testis. UGT1A10 was mainly expressed in gastrointestinal tract and bladder and marginally expressed in liver, kidney, ovary, and uterus. UGT2B4 was highly expressed in liver, moderately in breast, and marginally in the other tissues. UGT2B7 was expressed in all tissues, highly in liver, small intestine, colon, kidney, bladder, and uterus. UGT2B10 was highly expressed in liver and bladder. UGT2B11 was highly expressed in liver, bladder, and breast and moderately in kidney and uterus. UGT2B15 was highly expressed in liver, gastrointestinal tract, bladder, breast, ovary, and testis. UGT2B17 was highly expressed in liver, stomach, colon, and testis. UGT2B28 was highly expressed in bladder and marginally in liver, stomach, and breast.
RT-PCR analyses of UGT mRNA in human normal tissues. Total RNA samples from human normal tissues were analyzed by RT-PCR using primers specific for each UGT isoform.
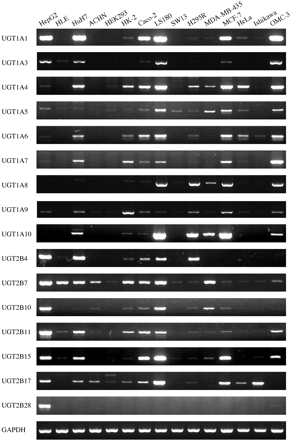
UGT mRNA Expression in Human Tissue-Derived Cell Lines. The expression of UGT mRNA in various human tissue-derived cell lines is shown in Fig. 2. UGT1A1 was highly expressed in HepG2, HuH7, Caco-2, LS180, MCF-7, and OMC-3 cells and marginally in HK-2, H295R, MDA-MB-435, and HeLa cells. UGT1A3 was detected in HepG2, HLE, HuH7, Caco-2, LS180, MCF-7, and OMC-3 cells. The expression profile of UGT1A4 was similar to that of UGT1A1. UGT1A5 was highly expressed in LS180, MDA-MB-435, MCF-7, and OMC-3 cells. UGT1A6 was detected in HepG2, HuH7, HK-2, Caco-2, LS180, H295R, MCF-7, HeLa, Ishikawa, and OMC-3 cells. The expression profiles of UGT1A7 and UGT1A9 were nearly identical to that of UGT1A6. UGT1A8 was highly expressed in LS180, H295R, MDA-MB-435, MCF-7, and OMC-3 cells. UGT1A10 was highly expressed in LS180 and MCF-7 cells, followed by H295R, MDA-MB-435, HuH7, and OMC-3 cells. UGT2B4 was highly expressed in HepG2 and HuH7, followed by H295R, LS180, Caco-2, and HK-2 cells. UGT2B7 was expressed in all cell lines. UGT2B10 was highly expressed in HepG2, LS180, and MDA-MB-435 cells. UGT2B11 was highly expressed in HepG2, HuH7, HK-2, Caco-2, LS180, and OMC-3 cells. UGT2B15 was highly expressed in HepG2, HuH7, Caco-2, LS180, MCF-7, and OMC-3 cells. The UGT2B17 mRNA was highly expressed in HepG2, LS180, MCF-7, and Ishikawa cells. UGT2B28 was expressed only in HepG2 cells.
RT-PCR analyses of UGT mRNA in human tissue-derived cell lines. Total RNA samples from various cell lines were analyzed by RT-PCR using primers specific for each UGT isoform.
Discussion
We determined the expression levels of the functional UGT1A and UGT2B isoforms in human normal tissues and human tissue-derived cell lines. Because of the limited availability of antibodies that are specific for each UGT isoform as well as overlapping substrate specificities, it was difficult to evaluate the protein level. In contrast, the mRNA levels for each isoform can be specifically determined by RT-PCR using specific primers. For this reason, we determined the UGT mRNA levels.
The expression profiles of the UGT1A and UGT2B isoforms in liver, gastrointestinal tract, and kidney were largely consistent with those of previous studies (King et al., 2000; Tukey and Strassburg, 2000). The UGTs in these tissues would contribute to the first-pass effects and clearance of drugs, as a previous study (Soars et al., 2002) demonstrated that the microsomes from these tissues had certain abilities of glucuronidation. In disagreement with previous studies (Ritter et al., 1992; Tukey and Strassburg, 2000), the present study detected the expression of UGT1A1 in kidney and UGT1A10 in liver, although the levels were extremely low (Fig. 1). This discrepancy may be due to interindividual differences in the UGT expression. Additionally, differences in the experimental conditions in PCR and/or the primers between the present and previous studies may also have affected the results.
This is the first study to determine comprehensively the mRNA expression of each UGT isoform in human lung, bladder, and steroid-related tissues. The UGTs were hardly expressed in lung in this study. Meanwhile, all UGT1A and UGT2B isoforms except UGT2B17 were expressed in bladder. Giuliani et al. (2005) have reported that UGT1A protein was detected in normal bladder by immunohistochemistry. Since the bladder is exposed to numerous xenobiotics, UGTs expressed in bladder would also contribute to the detoxification of xenobiotics, possibly participating in the protection from toxins. Our finding of the substantial expression of UGTs in bladder may provoke researchers to investigate the glucuronidation capabilities of bladder microsomes. We found that the UGTs were also expressed in steroid-related tissues in isoform-specific manners. However, the present study unfortunately could not determine the interindividual variability of the expression levels of UGTs, because the RNA samples we used were from an individual sample or pooled samples. Strassburg et al. (2000) reported that the UGT1A and UGT2B isoforms were present in gastrointestinal tissues from some individuals but were absent in those from other individuals. The interindividual differences in the expression may partly result from genetic polymorphisms, because genetic polymorphisms on the promoter or coding region affect the transcriptional activity or mRNA stability. Actually, in the UGT2B17 gene, a deletion allele has been reported (Wilson et al., 2004). Although we did not determine the genotype of UGT2B17 in our samples, polymorphisms may affect the variability of UGT2B17 expression. In addition to genetic polymorphisms, the induction by environmental and/or dietary factors or differences in the levels of transcriptional factors would be causal factors of the variability of the UGT expression. It would be worth elucidating the interindividual variability in UGT expression in steroid-related tissues and bladder. Finally, previous immunohistochemical studies demonstrated that UGTs localize in certain cell type or specific region in tissues such as small intestine (Strassburg et al., 2000), kidney (Gaganis et al., 2007), bladder (Giuliani et al., 2005), breast (Gestl et al., 2002), and uterus (Lépine et al., 2004). However, the studies with total RNA from whole tissue cannot specify the expression in a specific cell type. We should keep this point in mind to predict or extrapolate the role of the expressed UGTs on metabolism of drugs, carcinogens, and endogenous compounds.
The expression profiles of UGT mRNA in the human tissue-derived cell lines were not necessarily consistent with those in corresponding normal tissues. HLE cells showed no detectable UGT1A expression, albeit they are derived from liver. Although the reasons remain unclear, HLE cells would not be suitable for the study of UGT1A expression. In LS180 cells that are derived from colon, all UGT1A and UGT2B isoforms except for UGT2B28 were expressed. This cell line might be an appropriate tool for in vitro studies of the expression and regulation of UGTs. It was interesting that MCF-7 cells, which are estrogen receptor-positive (Guthrie et al., 1997) breast epithelial adenocarcinoma cells, expressed all UGT1A isoforms, but MDA-MB-435 cells, which are estrogen receptor-negative (Guthrie et al., 1997) breast ductal carcinoma cells, expressed limited kinds of UGT1A isoforms. It was also interesting that the expression levels of UGT2B7 and UGT2B10 were higher in MDA-MB-435 cells than in MCF-7 cells, but those of UGT2B15 and UGT2B17 were higher in MCF-7 cells than in MDA-MB-435 cells. Collectively, the expression profiles of UGT mRNA in the cell lines show diversity, although the derived organs were the same. The information presented here would be useful for choosing suitable cell lines for in vitro studies on UGTs.
In summary, we comprehensively determined the expression profiles of UGT1A and UGT2B in human normal tissues and human tissue-derived cell lines. The findings will be useful for understanding the physiological significance of each UGT isoform and to predict the capabilities of glucuronidation in various tissues. In addition, our results provide basic information on UGT expression in various kinds of cell lines.
Acknowledgments
We acknowledge Brent Bell for reviewing the manuscript.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.108.021428.
-
ABBREVIATIONS: UGT, UDP-glucuronosyltransferase; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcriptase-polymerase chain reaction.
-
↵
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material. - Received March 11, 2008.
- Accepted May 13, 2008.
- The American Society for Pharmacology and Experimental Therapeutics