Visual Overview
Abstract
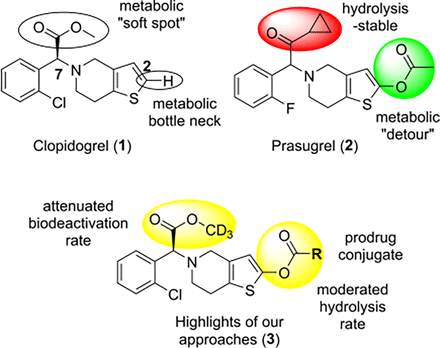
The delicate balance between ischemic and bleeding risks is a critical factor in antiplatelet therapy administration. Clopidogrel and prasugrel, belonging to the thienopyridine class of antiplatelet drugs, are known for their variability in individual responsiveness and high incidence of bleeding events, respectively. The present study is centered on the development and assessment of a range of deuterated thienopyridine derivatives, leveraging insights from structure-pharmacokinetic relationships of clopidogrel and prasugrel. Our approaches were grounded in the molecular framework of clopidogrel and incorporated the C2-pharmacophore design from prasugrel. The selection of ester or carbamate substituents at the C2-position facilitated the generation of the 2-oxointermediate through hydrolysis, akin to prasugrel, thereby bypassing the issue of CYP2C19 dependency. The bulky C2-pharmacophore in our approach distinguishes itself from prasugrel’s acetyloxy substituent by exhibiting a moderated hydrolysis rate, resulting in a more gradual formation of the active metabolite. Excessive and rapid release of the active metabolite, believed to be linked with an elevated risk of bleeding, is thus mitigated. Our proposed structural modification retains the hydrolysis-sensitive methyl ester of clopidogrel but substitutes it with a deuterated methyl group, shown to effectively reduce metabolic deactivation. Three promising compounds demonstrated a pharmacokinetic profile similar to that of clopidogrel at four times the dose, while also augmenting its antiplatelet activity.
SIGNIFICANCE STATEMENT Inspired by the structure-pharmacokinetic relationship of clopidogrel and prasugrel, a range of clopidogrel derivatives were designed, synthesized, and assessed. Among them, three promising compounds have been identified, striking a delicate balance between efficacy and safety for antiplatelet therapy. Additionally, the ozagrel prodrug conjugate was discovered to exert a synergistic therapeutic effect alongside clopidogrel.
Footnotes
- Received February 24, 2024.
- Accepted May 6, 2024.
This study was supported by the Scientific and Technological Development Plan of Jilin Province China (Grant 20210204068YY).
No author has an actual or perceived conflict of interest with the contents of this article.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
- Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics