Abstract
In pregnant women, CYP3A activity increases by 100% during the third trimester (T3). Due to logistical and ethical constraints, little is known about the magnitude of CYP3A induction during the first trimester (T1) and second trimester (T2). Our laboratory has shown that sandwich-cultured human hepatocytes (SCHH) and HepaRG cells have the potential to predict the magnitude of in vivo induction of CYP3A activity likely to be observed in T1 and T2. Therefore, we incubated SCHH and HepaRG cells with plasma concentrations of various pregnancy-related hormones (PRHs)—individually or in combination—observed during T1, T2, or T3 in pregnant women. Then, CYP3A activity was measured by 1′-OH-midazolam formation. In all three trimesters, only cortisol (C) consistently and significantly induced CYP3A activity, while other individual hormones (progesterone, estradiol, or growth hormones) failed to induce CYP3A activity. At physiologically relevant 1× plasma concentrations, the magnitude of CYP3A induction by C or the combination of all PRHs did not change significantly with gestational age. The pattern of induction of CYP3A activity in SCHH by the hormones was similar to that in HepaRG cells. Based on these data, we conclude that C remains the major inducer of CYP3A activity earlier in gestation. Moreover, we predict that the magnitude of CYP3A induction during T1 and T2 will be similar to that observed during T3 (∼100% increase versus postpartum). This prediction is consistent with the observation of similar increases in T2 and T3 oral clearance of indinavir (a CYP3A cleared drug) versus postpartum.
Introduction
Despite the general apprehension surrounding the use of drugs during pregnancy, a pregnant woman is frequently prescribed medication to treat a variety of preexisting chronic conditions (e.g., HIV infection, epilepsy, hypertension, solid organ transplantation, etc.), acute conditions (e.g., influenza), or pregnancy-related conditions (e.g., nausea, gestational diabetes, or hypertension). If left untreated, these conditions could have deleterious consequences to the mother and/or her fetus. Therefore, it is not surprising that between 2006 and 2008, about 82% of pregnant women took medication sometime during pregnancy and about 50% took medication during the first trimester (T1) (Mitchell et al., 2011).
Pregnancy is associated with a myriad of physiologic changes including hepatic metabolism (Abduljalil et al., 2012). Among these is approximately a 2-fold induction of CYP3A activity during the third trimester (T3), as measured by 1′-OH-midazolam formation clearance (Hebert et al., 2008). Based on genotyping data and modeling and simulation, we have shown that this induction of CYP3A activity is primarily due to induction of hepatic CYP3A4 activity (Hebert et al., 2008; Ke et al., 2012). Many drugs routinely prescribed to pregnant women are cleared by CYP3A enzymes, such as protease inhibitors (e.g., ritonavir), antihypertensive drugs (e.g., nifedipine), and hypoglycemics (e.g., glyburide). The magnitude of induction in CYP3A activity observed during T3 can result in subtherapeutic plasma concentrations of drugs such as in the HIV drug indinavir (Unadkat et al., 2007). Logistical and ethical constraints make it difficult to conduct prospective mechanistic studies in pregnant women using validated CYP3A substrates, especially in the T1 or second trimester (T2). As a result, the magnitude of induction of CYP3A activity during T1 or T2 is not known. This lack of information makes it difficult for clinicians to design dosing regimens of CYP3A cleared drugs during T1 or T2.
The plasma concentrations of several pregnancy-related hormones (PRHs) rise substantially as gestation proceeds (Table 1). Our laboratory has previously demonstrated that a cocktail of PRHs [the hormone combination containing cortisol (C), progesterone (P), 17β-estradiol (E2), and growth hormone (GH) plus placental growth hormone (PGH) (GH-PGH); hereafter referred to as the PRH cocktail], at their T3 plasma concentrations observed in pregnant women, induced CYP3A activity in sandwich-cultured human hepatocytes (SCHHs) to an extent comparable to that observed in vivo during T3. Of all these hormones, cortisol was the most potent inducer of CYP3A activity in SCHHs. Additionally, the induction pattern observed in SCHHs was replicated in HepaRG cells (Papageorgiou et al., 2013). Both these in vitro models demonstrated their potential to predict the magnitude of CYP3A induction in early pregnancy. Therefore, we hypothesized that the magnitude of in vivo hepatic CYP3A induction during T1 and T2 can be quantitatively predicted from studies in SCHH or HepaRG cells exposed to PRHs at plasma concentrations observed in pregnant women (for brevity, referred to as concentrations hereafter) during T1 and T2. The studies presented here were conducted in HepaRG and SCHHs to examine the temporal relationships between concentrations of PRHs and induction of CYP3A activity over the course of pregnancy, and, based on these data, to predict the magnitude of induction in CYP3A activity during T1 and T2.
Plasma concentrations of PRHs observed in pregnant women during T1, T2, or T3 and targeted to use in this study after correcting for depletion
Materials and Methods
Chemicals and Reagents.
Growth hormone, 17β-estradiol, cortisol, progesterone, and diazepam were purchased from Sigma-Aldrich (St. Louis, MO). Placenta growth hormone was obtained from GenWay Biotech, Inc. (San Diego, CA). Midazolam, 1-OH-midazolam, 1-OH-midazolam-d4, and E2-d5 were purchased from Cerilliant Corporation (Round Rock, TX). Acetic acid (American Chemistry Society grade), acetonitrile (mass spectrometry grade) were obtained from Fisher Scientific (Pittsburgh, PA). William’s Medium E, Dulbecco’s phosphate-buffered saline, GlutaMax-I, penicillin-streptomycin (10,000 U/ml), and insulin-transferrin-selenium were purchased from Life Technologies (Carlsbad, CA). Matrigel (growth factor reduced, phenol red free) was obtained from BD Biosciences (San Jose, CA).
Hormone Depletion in HepaRG Cells or SCHH.
In-house differentiated HepaRG cells or SCHH in 96-well plates were incubated (in triplicate or duplicate, respectively) with varying concentrations of PRHs (Table 3). Media were sampled at various time points up to 12 hours. To quantify cortisol or progesterone, media samples (15 µl) were protein precipitated with 85 µl acetonitrile containing diazepam (internal standard, 10 ng/ml) and centrifuged at 3000g for 15 minutes prior to liquid chromatography–tandem mass spectrometry analysis. To quantify estradiol, media samples (15 µl) were spiked with 15 µl estradiol-d5 (internal standard, 100 ng/ml in 10% acetonitrile), extracted with 800 µl methyl tertiary butyl ether, and then centrifuged at 3000g for 15 minutes. The organic phase was evaporated under nitrogen. The dried residue was reconstituted and derivatized using dansyl chloride to enhance electrospray ionization following the procedure described previously (Kushnir et al., 2008).
Depletion half-lives of steroid hormones when incubated with HepaRG cells or SCHH
The aforementioned samples were analyzed by liquid chromatography–tandem mass spectrometry using an Agilent 1290 Infinity ultra-performance liquid chromatography (UPLC) system coupled to an Agilent 6400Triple Quad mass spectrometer (Agilent Technologies, Santa Clara, CA). An Acquity UPLC BEH, ethylene bridged hybrid; (BEH) C18 Column (1.7 µm, 2.1 mm × 50 mm; Waters Corporation, Milford, MA) was used with the mobile phase consisting of 0.1% acetic acid (v/v) in water (phase A) or 0.1% acetic acid (v/v) in acetonitrile (phase B) (total flow rate 0.25 ml/min−1). For the cortisol and progesterone assay, the following linear gradient was used: 80% mobile phase A, 0–0.3 minutes; 80%–20%, 0.3–1.5 minutes; 20%, 1.5–2.5 minutes; 20–80%, 2.5–2.6 minutes; and 20%, 2.6–4 minutes. The multiple reaction monitoring pairs selected for cortisol, progesterone, and diazepam were 363.2/121.1, 315.2/97.1, and 285/193, respectively. For the estradiol assay, the following gradient was chosen: 50% mobile phase A, 0–0.1 minutes; 50%–5%, 0.1–0.7 minutes; 95%, 0.7–2.5 minutes; 95%–50%, 2.5–2.6 minutes; and 50%, 2.6–4 minutes. The multiple reaction monitoring pairs selected for dansyl-estradiol and dansyl-estradiol-d5 were 506.1/171.1 and 511/171.1, respectively.
Hormonal Treatment of HepaRG Cells or SCHH.
Proliferative state HepaRG cells were kindly provided by Biopredic International (Overland Park, KS). The cells were in-house expanded and differentiated according to the provider’s protocols. Differentiated HepaRG cells were cultured in 96-well plates at a density of ∼0.8 million cells/ml. Since the commercially available HepaRG maintenance and induction medium supplements contain hydrocortisone succinate, a CYP3A inducer, we conducted induction studies in the absence of hydrocortisone succinate to avoid confounding interpretation of our data (Papageorgiou et al., 2013). In brief, the differentiated HepaRG cells were maintained for 72 hours in William’s Medium E supplemented with GlutaMAX-I, insulin-transferrin-selenium, and penicillin-streptomycin. At the end of the maintenance period, the medium was removed and the cells were treated with the control [a cocktail of C, GH, E2, and P at their unbound concentrations observed in nonpregnant women; hereafter referred to as the control (CTRL)] or various PRHs individually or in combination at unbound, 1×, or 10× T1, T2, or T3 concentrations (Table 1). Because some of the hormones were depleted, the actual incubation concentrations were adjusted, based on their half-life of depletion (Table 3), such that the AUC/τ of the hormone media concentration approximated the values listed in Table 1. In addition, to compensating for this depletion, during the 72 hour induction period with the PRHs, induction media were renewed every 12 hours (see Results). When comparing CYP3A activity by T1, T2, or T3 concentrations, these experiments were conducted on the same day with the same batch of HepaRG cells or SCHH. In all the experiments, a positive control [10 µM rifampin (RIF)] and a vehicle control were included. All incubations contained 0.1% pH 9 deionized water and 0.9% methanol. CYP3A activity in these HepaRG cells was determined as described subsequently. Except for CTRL (n = 6), the experiments were performed in triplicate.
Cryopreserved human hepatocytes from three premenopausal donors (Table 2) were either commercially procured (Hu1587 and Hu1595; Life Technologies) or provided gratis (Hu4059; Research Triangle Labs, Research Triangle, NC). Hepatocytes were plated in collagen-coated 96-well plates at a density of ∼0.7 million viable cells/ml and overlaid with Matrigel (BD Biosciences) according to the manufacturer’s instructions. Since the hepatocyte medium supplements normally contain dexamethasone, a well-documented glucocorticoid CYP3A inducer, we omitted the addition of dexamethasone to the maintenance and induction media. At the end of the 72 hour maintenance period, the SCHHs were treated (including change of media) as described previously for the HepaRG cells. CYP3A activity in these SCHHs was determined as described subsequently. All experiments were performed in triplicate.
Demographic information of the hepatocyte donors
Cortisol Concentration-CYP3A Activity Induction Relationship in SCHH.
The SCHHs (batch Hu1587) in collagen-coated 96-well plates were maintained in dexamethasone-free medium for 72 hours and then treated with CTRL treatment or various concentrations of cortisol, ranging from T1 unbound concentration to 50× T3 concentrations as described previously (concentrations were adjusted, where appropriate, to compensate for depletion). CYP3A activity in these SCHHs was determined as described subsequently. All treatments were performed in duplicate except for the unbound cortisol concentrations and CTRL treatments (n = 3).
CYP3A Activity Assay.
At the end of the induction period, induction media were aspirated, HepaRG cells or SCHH were rinsed twice with prewarmed Dulbecco’s phosphate-buffered saline (∼150 µl) and incubated for 60 minutes (HepaRG cells) or 20 minutes (SCHH) with 2µM midazolam dissolved in serum-free William’s Medium E. Then, an equal volume of ice-cold acetonitrile containing 10 nM 1′-OH-midazolam-d4 (internal standard) was immediately added to the supernatant and assayed by liquid chromatography–tandem mass spectrometry as described previously (Shirasaka et al., 2013).
Statistical and Data Analysis.
Data are expressed as mean ± S.D. values unless otherwise stated. One-way analysis of variance, followed by post hoc tests (Dunnett’s test when the PRH treatment groups were compared with the CTRL treatment or Tukey’s test when the PRH treatment groups were compared with each other), were performed. The Emax and EC50 of induction of CYP3A activity by cortisol was estimated by fitting the simple Emax or the sigmoid Emax model to the concentration-response data using nonlinear regression (Graphpad Prism 5.0, La Jolla, CA). The baseline value of induction (E0) was fixed to 1. The model with the smaller Akaike information criterion value was selected (Ludden et al., 1994).
Results
Hormone Depletion when Incubated with HepaRG or SCHH.
In HepaRG cells, cortisol at the unbound concentration observed in nonpregnant women was depleted with a half-life of ∼13 hours, while at T3 1× and 10× concentrations, cortisol was not depleted (Table 3). In contrast, estradiol and progesterone were more rapidly depleted. To compensate for this depletion, the induction media in all future experiments (including SCHH) were renewed every 12 hours and the concentrations of the hormones in the media were adjusted such that the average concentration (AUC/τ) approximated the corresponding average concentrations during pregnancy. GH-PGH depletion was not measured because an assay to measure the depletion of PGH is currently not available.
Induction of CYP3A Activity in HepaRG Cells by Cortisol or Cortisol plus Other PRHs.
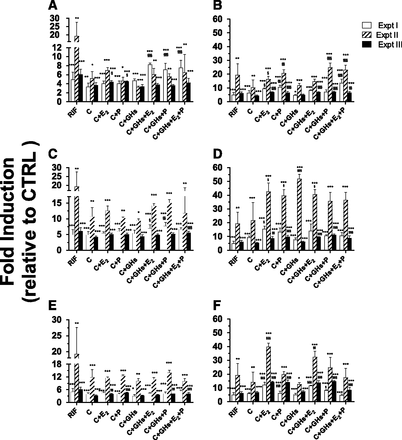
A pilot study using T3 unbound, 1×, or 10× concentrations of individual PRHs was conducted. Except for cortisol, none of the PRHs induced CYP3A activity to a significant extent (Fig. 1). Therefore, only cortisol and cortisol containing PRH combinations were included in all subsequent experiments described subsequently (including SCHH).
CYP3A activity in HepaRG cells incubated with T3 plasma concentrations of individual PRHs observed in pregnant women or 10 µM RIF. Fold induction (mean ± S.D.; n = 3) is expressed relative to control treatment (CTRL, n = 6; unbound plasma concentrations of C + GH + E2 + P observed in nonpregnant women). Data were analyzed by one-way analysis of variance followed by Dunnett’s test. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with CTRL.
As observed previously, cortisol or cortisol plus other hormones consistently, significantly, but variably induced CYP3A activity at 1× or 10× (but not at the unbound) concentrations, compared with the CTRL treatment (Fig. 2). At 10× concentrations, treatments containing C + E2 or C + P induced CYP3A activity greater than C alone (Fig. 2, B, D, and E). However, this greater induction was not consistently present in treatments containing GH-PGH. Our positive control, 10 µM RIF, significantly induced CYP3A activity (range: 5–19-fold), whereas CYP3A activity in the presence of the vehicle control was not significantly different from CTRL (data not shown).
CYP3A activity in HepaRG cells incubated with T1 (A and B), T2 (C and D), or T3 (E and F) at 1× (A, C, and E) or 10× (B, D, and F) plasma concentrations of PRHs observed in pregnant women or 10 µM RIF. Fold induction (mean ± S.D.; n = 3) is expressed relative to control treatment (CTRL, n = 6; unbound plasma concentrations of C + GH + E2 + P observed in nonpregnant women). Data were analyzed by one-way analysis of variance followed by Dunnett’s test. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with CTRL. §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 compared with cells exposed to C alone.
Gestational Age–Dependent Induction of CYP3A Activity in HepaRG Cells by Cortisol or PRH Cocktail.
The magnitude of induction of CYP3A activity was not significantly different across the three trimesters at 1× concentrations of the hormones. At 10× concentrations, significant differences emerged (Fig. 3D). The PRH cocktail resulted in varying temporal patterns in different batches at 10× concentrations. No consistent cortisol or PRH cocktail concentration-dependent induction in CYP3A activity was observed.
CYP3A activity in HepaRG cells incubated with 1× or 10× plasma concentrations of cortisol (A and C, respectively) or PRH cocktail (B and D, respectively) observed in pregnant women. Fold induction (mean ± S.D.; n = 3) is expressed relative to control treatment (CTRL, n = 6; unbound plasma concentrations of C + GH + E2 + P observed in nonpregnant women). Data were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison to detect any difference in the magnitude of CYP3A induction among T1, T2, and T3. §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 compared with the indicated trimester.
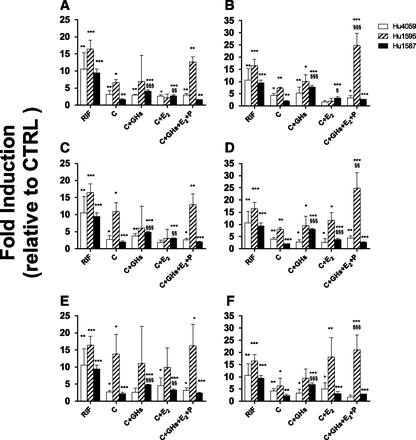
Induction of CYP3A Activity in SCHH by Cortisol or Cortisol plus Other PRHs.
Similar to the HepaRG cells, the cortisol and PRH cocktail consistently, variably, and significantly induced CYP3A activity in SCHH at 1× and 10× concentrations (and in one out of three donors at the unbound concentration, data not shown). In batch Hu1587 alone, treatments containing C + GH-PGH or C + E2 induced CYP3A activity greater than C alone (Fig. 4, A–E). In all three batches, 10 µM RIF significantly induced CYP3A activity (range: 9–16-fold). CYP3A activity in the vehicle control was not significantly different from that observed in CTRL.
CYP3A activity in SCHH incubated with T1 (A and B), T2 (C and, D), or T3 (E and F) at 1× (A, C, and E) or 10× (B, D, and F) plasma concentrations of PRHs observed in pregnant women or 10 µM RIF. Fold induction (mean ± S.D.; n = 3) is expressed relative to control treatment (CTRL; unbound plasma concentrations of C + GH + E2 + P observed in nonpregnant women). Data were analyzed by one-way analysis of variance followed by Dunnett’s test. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with CTRL. §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 compared with SCHH exposed to C alone.
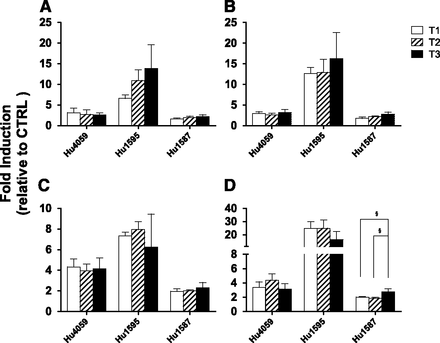
Gestational Age–Dependent Induction of CYP3A Activity in SCHH by Cortisol or PRH Cocktail.
As in the HepaRG cells, at 1× concentrations the induction of CYP3A in SCHH was independent of gestational age. At 10× concentrations, CYP3A induction was similar throughout all three trimesters, except that in one batch (Hu1587) CYP3A activity was modestly increased (∼30%) by the PRH cocktail in T3 versus earlier trimesters.
Concentration-Dependent Induction of CYP3A Activity in SCHH by Cortisol.
Cortisol induced CYP3A activity in SCHH (batch Hu1587) in a concentration-dependent manner. The simple Emax model rather than the sigmoid Emax model was considered to be the best model for the data based on the Akaike information criterion value (−43.624 and −35.716, respectively). At cortisol plasma concentrations spanning from T1 to T3, the induction of CYP3A activity reached a plateau and remained constant.
Discussion
Previously, we have shown that of all the PRHs studied, cortisol was the major inducer of CYP3A activity in HepaRG cells and SCHH at T3 concentrations (Papageorgiou et al., 2013). Consistent with these findings, cortisol remained the major inducer in HepaRG cells at T1 or T2 concentrations (Figs. 2 and 3). Therefore, all subsequent studies examined the induction of CYP3A activity by cortisol alone or in combination with other PRHs. We chose to use the combination of GH and PGH in our incubations because both are present during pregnancy. The plasma concentration of GH decreases while that of PGH increases as pregnancy proceeds (Fuglsang and Ovesen, 2006). In addition, these two hormones interact with the same receptor with similar affinity (Baumann et al., 1991) and trigger the same intracellular signaling pathways (Silva et al., 2002). Since some of the hormones were rapidly depleted when incubated with HepaRG cells or SCHH, we adjusted the incubation concentrations of the hormones and frequency of change of media to account for this depletion.
Induction of CYP3A activity by cortisol or PRHs in HepaRG cells or SCHH was quite variable from batch to batch. The source of this variability is not clear but may be due to varying percentages of hepatocyte-like cells versus biliary cells between the different batches and passages in our in-house differentiated HepaRG cells (Schulze et al., 2012). Interestingly, in general, the batch of cells demonstrating the highest CYP3A induction by RIF also exhibited the highest CYP3A induction by cortisol alone or in combination. However, this variability in induction of CYP3A activity by cortisol (or PRHs) cannot be quantitatively explained by induction in CYP3A activity by RIF. For example, in SCHH, at T3 1× concentration, cortisol induced CYP3A activity in batch Hu4059 ∼4-fold more than in batch Hu1595. In contrast, RIF induced CYP3A activity in batch Hu4059 only ∼50% more than that in batch Hu1595.
Incubation of HepaRG cells or SCHH with the unbound concentrations of PRHs observed during T1, T2, or T3 did not result in induction of CYP3A activity. Therefore, only the 1× and 10× concentration data are shown in Figs. 2–5. Although there was some batch-to-batch variability; in general, the induction of CYP3A activity in HepaRG cells at 1× concentrations of cortisol alone was comparable to that when cortisol was combined with other PRHs (Fig. 2, A, C, and E). Of note, at 10× concentrations, induction of CYP3A activity with C + E2 or C + P was greater than with C alone. However, this was not always the case when induction in CYP3A activity by the triple combinations of hormones (e.g., C + GH-PGH + P) or the PRH cocktail was compared with C alone.
CYP3A activity in SCHH incubated with 1× or 10× plasma concentrations of cortisol (A and C, respectively) or PRH cocktail (B and D, respectively) observed in pregnant women. Fold induction (mean ± S.D.; n = 3) is expressed relative to control treatment (CTRL, n = 3; unbound plasma concentrations of C + GH + E2 + P observed in nonpregnant women). Data were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison to detect any difference in the magnitude of CYP3A induction among T1, T2, and T3. §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 compared with the indicated trimester.
Based on these observations and due to our previous data in SCHHs on C + GH-PGH demonstrating greater induction of CYP3A activity than with C alone (Papageorgiou et al., 2013), the number of hormonal combination treatments examined in SCHH was reduced. Similar to HepaRG cells, despite interbatch variability, the magnitude of induction of CYP3A activity by dual hormone treatments or PRH cocktail was in general comparable to that by C alone at both 1× and 10× concentrations. One batch of SCHH (Hu1587) exhibited a modestly greater induction of CYP3A activity by the dual hormone treatments (C + E2 or C + GH-PGH) compared with C alone at 1× and 10× concentrations. At T1, T2, or T3 10× concentrations, the PRH cocktail induced CYP3A activity greater than with C alone.
The aforementioned observed pattern of induction is consistent with our previous observations where cortisol alone was the major inducer of CYP3A activity in HepaRG cells or SCHH (Papageorgiou et al., 2013). This work, together with our previous report, provides strong evidence that cortisol is a major inducer of CYP3A activity throughout pregnancy. Indeed, unequivocal data exist indicating that glucocorticoids induce CYP3A activity in human hepatocytes and in vivo (Watkins et al., 1989; Lu and Li, 2001). However, the mechanism(s) by which this induction occurs is not clear. Evidence suggests that at low plasma concentrations of glucocorticoids, these hormones induce CYP3A activity via the glucocorticoid receptor, while at supraphysiological concentrations, PXR is involved (Lehmann et al., 1998; Pascussi et al., 2000). Aside from the glucocorticoid receptor and PXR, multiple lines of evidence suggest that a PXR-independent mechanism(s) may be involved in induction of CYP3A activity (Schuetz et al., 2000; Xie et al., 2000; Zimmermann et al., 2009). Others have also reported enhanced induction of CYP3A activity by cortisol in the presence of GH (Thangavel et al., 2011). They attributed this to greater activation and nuclear translocation of transcriptional factors (i.e., HNF-4α and PXR) and enhanced binding of these factors to the CYP3A4 regulatory region (Thangavel et al., 2011). Previous studies have demonstrated that at supraphysiological concentrations estradiol or progesterone activates PXR (Handschin and Meyer, 2003; Mnif et al., 2007). However, this does not signify that estradiol or progesterone (or both) induces CYP3A activity at plasma concentrations observed in pregnant women. Indeed, consistent with our data, at these lower plasma concentrations of estradiol or progesterone, these hormones failed to consistently induce CYP3A activity in human hepatocytes (Choi et al., 2013).
Based upon the aforementioned data, we examined the induction of CYP3A activity by cortisol alone or by PRH cocktail at T1, T2, or T3 1× or 10× concentrations in both HepaRG cells and SCHH. Although the absolute magnitude of CYP3A induction varied between these two in vitro models, the induction of CYP3A activity by 1× concentrations of cortisol or PRH cocktail was independent of gestational age (Figs. 3 and 5). In contrast, at 10× concentrations, some significant differences manifested, but these differences were modest.
The plasma concentration of cortisol rises considerably as pregnancy proceeds. Cortisol plasma concentration increases from T1 to T2 by ∼2-fold and then remains constant during T3. However, this increase did not translate into a significant difference in induction of CYP3A activity (Fig. 3, A and B; Fig. 5, A and B). To gain insight into this observation, we determined the relationship between cortisol concentration and induction of CYP3A activity in one batch of SCHH (Fig. 6). The results show that at the total cortisol plasma concentration (181.5 nM) observed in nonpregnant women (Lindholm and Schultz-Möller, 1973; Maroulis et al., 1976; Kalleinen et al., 2008; Matsuzaka et al., 2013) or in pregnant women during T1, T2, or T3, the CYP3A activity would be predicted to be induced by 45%, 120%, 170%, and 170%, respectively. While the predicted induction in CYP3A activity during T1 appears numerically lower than that during T2 or T3, our experimental data show that this difference is not statistically significant (Figs. 3 and 5). This is because at 1× cortisol concentrations spanning T1–T3, the induction of CYP3A activity appears to be approaching a plateau. Based on these data, we predict that the magnitude of CYP3A induction during T1 or T2 will be similar to that during T3 (∼2-fold). Evidence in the literature supports our predictions. Although not an optimum CYP3A probe, the urinary dextromethorphan/3-OH-morphinan ratio remains constant throughout pregnancy (Tracy et al., 2005). Also, the clearance of CYP3A substrates, indinavir and nifedipine, during T2 is comparable to that observed during T3 (Marin et al., 2007; Cressey et al., 2013).
CYP3A activity in SCHH (Hu1587) incubated with various plasma concentrations of cortisol. Fold induction is expressed relative to control treatment (CTRL, n = 3; unbound plasma concentrations of C + GH + E2 + P observed in nonpregnant women). Data are expressed as individual data points. Emax and EC50 (estimate and 95% confidence interval) were estimated by fitting the simple Emax model to the data.
In summary, this work, together with our previous report, provides strong evidence that cortisol is the major inducer of CYP3A activity throughout pregnancy in HepaRG cells and SCHH. Moreover, we predict that the magnitude of induction in CYP3A activity during earlier trimesters will be about 2-fold, similar to that in T3. Although these predictions should ideally be validated with CYP3A probe studies, our predictions are consistent with the limited clinical data on the disposition of CYP3A cleared drugs (dextromethorphan, nifedipine, and indinavir) during T2 and T3. The mechanism(s) by which cortisol induces CYP3A activity remains unclear. Given the consistency between HepaRG cells and SCHH in the pattern of induction of CYP3A activity by PRHs, we propose that HepaRG cells can serve as a model to elucidate the molecular mechanism(s) by which cortisol induces CYP3A activity during pregnancy.
Acknowledgments
The authors thank Jenny Sager and Dr. Nina Isoherranen for facilitating the midazolam assay.
Authorship Contributions
Participated in research design: Zhang, Farooq, Prasad, Grepper, Unadkat.
Conducted experiments: Zhang, Farooq.
Contributed new reagents or analytic tools: Prasad.
Performed data analysis: Zhang.
Wrote or contributed to the writing of the manuscript: Zhang, Farooq, Prasad, Grepper, Unadkat.
Footnotes
- Received January 6, 2015.
- Accepted March 23, 2015.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant P01DA032507].
Abbreviations
- BEH
- ethylene bridged hybrid
- C
- cortisol
- CTRL
- control
- E2
- 17β-estradiol
- GH
- growth hormone
- P
- progesterone
- PGH
- placental growth hormone
- PRH
- pregnancy-related hormone
- RIF
- rifampin
- SCHH
- sandwich-cultured human hepatocytes
- T1
- first trimester
- T2
- second trimester
- T3
- third trimester
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics