Abstract
We clarified that major human cytochrome P450 (P450) enzymes were expressed in a chimeric mouse line established recently in Japan, in which the liver could be replaced by more than 80% with human hepatocytes. In this study, we investigated major human phase II enzymes such as UDP-glucuronosyltransferase (UGT), sulfotransferase (SULT), N-acetyltransferase (NAT), and glutathione S-transferase (GST) in the livers of chimeric mice by mRNA, protein, and enzyme activity using reverse transcription-polymerase chain reaction, Western blot analysis, and high-performance liquid chromatography, respectively. Human UGT, SULT, NAT, and GST mRNA were expressed in the liver of the chimeric mice, and UGT2B7, SULT1E1, SULT2A1, and GSTA1 proteins could be detected. The expression of mRNA and protein was correlated with the human albumin (hAlb) concentration in mouse blood, the replacement of which by human hepatocytes could be estimated by the hAlb concentration in the blood of the chimeric mice, because the chimeric mice produce human albumin. The enzyme activities, such as morphine 6-glucuronosyltransferase activity and estrone 3-sulfotransferase activity, activities that are specific to humans but not to mice, were increased in a hAlb concentration-dependent manner. The chimeric mice with humanized liver with nearly 90% replacement by human hepatocytes demonstrated almost the same protein contents of human phase II enzymes and enzyme activities as those of the donor. In conclusion, the chimeric mice exhibited an efficient capacity of drug conjugation similar to that in humans. These chimeric mice expressed human phase II enzymes as well as P450s, suggesting that they could be a useful animal model in drug development.
Drug metabolism, drug interactions involving drug-metabolizing enzymes, and genetic polymorphisms of such enzymes have been well studied. To clarify the mechanism of the changed pharmacokinetics in studies of drug interactions, we usually focus on cytochrome P450 (P450) enzymes. However, a major metabolic pathway of a compound with polar functional groups is sometimes a conjugation reaction. The contribution of phase II conjugation to the clearance of a drug was estimated to be approximately 30% or higher (Bjornsson et al., 2003). Major hepatic phase II enzymes in humans are UDP-glucuronosyltransferase (UGT), sulfotransferase (SULT), N-acetyltransferase (NAT), and glutathione S-transferase (GST). There have been few reports on drug interactions that focused on phase II enzymes. Recently, several advances in the understanding of the inhibition and induction of such phase II enzymes have been made, especially concerning UGT. The effects of many drugs on glucuronidation in humans were clarified and listed by Kiang et al. (2005). Moreover, genetic polymorphisms of UGT, NAT, SULT, and GST have been demonstrated and the allelic frequency and effects of such mutations toward enzyme activities have become clearer. The UGT1A1*28 allele showed reduced UGT1A1 enzyme activity and was suggested to be a significant risk factor in the toxicity of irinotecan (Ando et al., 2000). The slow acetylator phenotype of NAT2 caused by the genetic polymorphism accounted for the increase in adverse reactions by isoniazid (Hughes et al., 1954). The combined GSTM1 and GSTT1 null genotypes showed correlations with the hepatotoxicity by troglitazone and tacrine (Simon et al., 2000; Watanabe et al., 2003). Attention should be given to the changes in pharmacokinetics caused by inhibition, induction, and genetic polymorphisms of phase II enzymes as well as P450s.
We previously clarified the expression of human P450s in the liver of chimeric mice with humanized liver, which were established using the urokinase-type plasminogen activator (uPA)/SCID mice by Tateno et al. (2004). The livers of such chimeric mice could be replaced by more than 80% with human hepatocytes (Tateno et al., 2004). In the present study, the purpose was to clarify the expression of human phase II enzymes such as UGT, SULT, NAT, and GST in these chimeric mice.
Materials and Methods
Materials. Rabbit anti-human UGT1A1 antibodies and rabbit anti-human UGT2B7 antibodies were purchased from BD Gentest (Woburn, MA). Rabbit anti-human SULT1E1 antibodies, rabbit anti-human SULT2A1 antibodies, and rabbit anti-human GSTA1 antibodies were obtained from Oxford Biomedical Research (Oxford, MI). Morphine hydrochloride was purchased from Takeda Chemical Industries (Osaka, Japan). Morphine 6-glucuronide was kindly provided by Dr. Kazuta Oguri (Kyusyu University, Fukuoka, Japan). UDP-glucuronic acid, alamethicin, estrone, estrone 3-sulfate, adenosine 3′-phosphate 5′-phosphosulfate (PAPS), sulfamethazine, acetylcarnitine, and carnitine acetyltransferase were obtained from Sigma-Aldrich (St. Louis, MO). Troglitazone, troglitazone glucuronide, and troglitazone sulfate were kindly provided by Sankyo (Tokyo, Japan). Pooled human liver microsomes (HLM), pooled human liver cytosol (HLC), and recombinant human UGT1A1 or UGT2B7 expressed in baculovirus-infected insect cells were obtained from BD Gentest. Recombinant human SULT1E1 or SULT2A1 expressed in baculovirus-infected insect cells and recombinant human GSTA1 produced from overexpresssing plasmid in Escherichia coli were obtained from Oxford Biomedical Research. All other chemicals and solvents were of the highest grade commercially available.
Generation of the Chimeric Mice with Humanized Liver. The present study was approved by the Ethics Committees of Kanazawa University and the Hiroshima Prefectural Institute of Industrial Science and Technology Ethics Board. The cryopreserved human hepatocytes from donor A (9-month-old white male) were purchased from In Vitro Technologies (Catonsville, MD). The human liver sample from donor B (12-year-old Japanese male) was obtained at autopsy after receiving written informed assent from the parents. The chimeric mice with humanized liver were generated by the method described previously (Tateno et al., 2004). The concentration of human albumin (hAlb) in the blood of the chimeric mice and the replacement index (RI; the rate of the replacement from mice to humans) were measured using ELISA and anti-human specific cytokeratin 8 and 18 antibody, respectively. There was a good correlation between the hAlb concentration and the RI (Tateno et al., 2004). The male chimeric mice used in this study were 11 to 14 weeks old (Table 1). The uPA+/+/SCID mice, uPA+/–/SCID mice, and uPA–/–/SCID mice were obtained as previously reported (Tateno et al., 2004).
Chimeric mice used in the present study
Hepatic RNA Extraction and Reverse Transcription-PCR. Human mRNA was quantified by TaqMan reverse transcription (RT)-PCR except for human SULT1E1. Human SULT1E1 mRNA was quantified by real-time RT-PCR. Total hepatic RNA was extracted using ISOGEN (Nippon Gene, Tokyo, Japan) and cDNAs were synthesized as described previously (Iwanari et al., 2002). The TaqMan RT-PCR and the real-time RT-PCR conditions were described previously by Nishimura et al. (2002) and Katoh et al. (2004), respectively. The sequences of primers and TaqMan probes used in this study were as follows. UGT1A1 (GenBank accession number NM_000463): sense primer (187–207) 5′-GACGCCTCGTTGTACATCAGA-3′, antisense primer (269–249) 5′-TCTTTCACATCCTCCCTTTGG-3′; TaqMan probe (209–238): 5′-ACGGAGCATTTTACACCTTGAAGACGTACC-3′; UGT2B7 (GenBank accession number NM_001074): sense primer (14–42) 5′-GGACTTCAGTAATTTTGCTAATACAACTG-3′, antisense primer (98–80) 5′-TATTCTGCTGCCCACACCA-3′; TaqMan probe (76–47): 5′-CCTTTCCACAATTCCCAGAGCTAAAGCAAA-3′; SULT1A1 (GenBank accession number NM_001055): sense primer (391–408) 5′-AACGCAAAGGATGTGGCA-3′, antisense primer (510–491) 5′-TCCGTAGGACACTTCTCCGA-3′; TaqMan probe (431–452): 5′-ACATGGCCAAGGTGCACCCTGA-3′; SULT1B1 (GenBank accession number NM_014465): sense primer (386–404) 5′-TGGCTCGTAATGCCAAGGA-3′, antisense primer (518–498) 5′-CAGGAACCATAGGCCACTTTT-3′; TaqMan probe (448–479): 5′-CAGCCTTTTCCTGGTACCTGGGAAGAATATCT-3′; GSTA1 (GenBank accession number S49975): sense primer (151–170) 5′-ATGTTCCAGCAAGTGCCAAT-3′, antisense primer (519–501) 5′-ACTGGAGTCAAGCTCCTCG-3′; TaqMan probe (331–361): 5′-GTATGTCCACCTGAGGAAAAAGATGCCAAGC-3′.
Other primers and probes were described previously (Nishimura et al., 2002), and the primers for human SULT1E1 (GenBank accession number NM_005420) by real-time PCR were as follows: sense primer (277–297) 5′-AAACAATTAGATGAGATGAAT-3′, antisense primer (450–433) 5′-ATTTGGATGACCAGCCAC-3′.
It was confirmed that the primer for human mRNA did not cross-react with murine mRNA at all using murine hepatic mRNA from control mice. The copy number of mRNA in the cDNA samples was calculated using standard amplification curves. We could not obtain total RNA from the donor B chimeric mice.
Liver Microsomes and Cytosol. Liver microsomes and cytosol were prepared as described previously (Yamazaki et al., 1999) and were stored at –80°C until analysis. The protein concentration was determined using Bradford protein assay reagent (Bio-Rad, Hercules, CA) with bovine gamma globulin as the standard. We could not obtain liver microsomes from donor A.
Immunoblot Analysis of Human Phase II Enzymes. SDS-polyacrylamide gel electrophoresis and immunoblot analysis of human UGT1A1, UGT2B7, SULT1E1, SULT2A1, and GSTA1 were performed according to the method of Laemmli (1970) with slight modifications. The liver microsomes (5–40 μg) were separated on 10% polyacrylamide gel for UGT and SULT and on 12% for GST, and transferred electrophoretically to a polyvinylidene difluoride membrane. Recombinant human UGTs, SULTs, and GST were also used as the standards. Biotinylated anti-rabbit IgG and a VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA) were used for diaminobenzidine staining.
Enzyme Assays. Morphine 6-glucuronosyltransferase activity was measured as described previously (Watanabe et al., 2002) with slight modifications. Briefly, a typical incubation mixture (total volume, 0.2 ml) contained 100 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (Tris)-HCl buffer (pH 7.4), 5 mM MgCl2, 25 μg of alamethicin/mg of microsomal protein, 3 mM UDP-glucuronic acid, 0.5 mg/ml HLM, and 1 mM morphine. The reaction was initiated by the addition of UDP-glucuronic acid and was then incubated for 60 min at 37°C. The reaction was terminated by adding 20 μl of ice-cold 70% perchloric acid. The product formation was determined using high-performance liquid chromatography (HPLC) with a C30 5-μm analytical column (Develosil, 4.6 × 150 mm; Nomura Chemical, Aichi, Japan). The mobile phase was 50 mM sodium dihydrogen phosphate and the flow rate was 1.5 ml/min. The eluent was monitored at 285 nm with a noise-base clean Uni-3 (Union, Gunma, Japan). Troglitazone sulfotransferase activity was measured as described previously (Honma et al., 2001) with slight modifications. Briefly, a typical incubation mixture (total volume, 0.2 ml) contained 50 mM Tris-HCl buffer (pH 7.4), 1 mM dithiothreitol, 100 μM PAPS, 0.5 mg/ml liver cytosol, and 1 μM troglitazone. The reaction was initiated by the addition of PAPS and was then incubated for 60 min at 37°C. The reaction was terminated by adding 200 μl of ice-cold acetonitrile. The product formation was determined using HPLC with a C18 5-μm analytical column (YMC-PACK, 4.6 × 150 mm; YMC, Kyoto, Japan). The mobile phase was 42% acetonitrile/0.05% phosphoric acid (v/v). The flow rate was 1.5 ml/min and the column temperature was 35°C. The eluent was monitored at 230 nm with a Uni-3. Estrone 3-sulfotransferase activity was measured as described previously (Suzuki et al., 2003) with slight modifications. Briefly, a typical incubation mixture (total volume, 0.2 ml) contained 50 mM Tris-HCl buffer (pH 7.4), 1 mM dithiothreitol, 7 mM MgCl2, 100 μM PAPS, 0.8 mg/ml liver cytosol, and 50 μM estrone. The reaction was initiated by the addition of PAPS and was then incubated for 60 min at 37°C. The reaction was terminated by adding 200 μl of ice-cold acetonitrile. The product formation was determined using HPLC with a C18 5-μm analytical column (Capcell Pak, 4.6 × 150 mm; Shiseido, Tokyo, Japan). The mobile phase was acetonitrile/67 mM Tris-HCl (pH 8.0) (25:75 v/v). The flow rate was 1.0 ml/min and the column temperature was 35°C. The eluent was monitored at 275 nm with a Uni-3. Sulfamethazine N-acetyltransferase activity was measured as described previously (Estrada-Rodgers et al., 1998) with slight modifications. Briefly, a typical incubation mixture (total volume, 0.2 ml) contained 50 mM Tris-HCl buffer (pH 7.4), 1 mM dithiothreitol, 5 mM acetylcarnitine, 0.1 U/ml carnitine acetyltransferase, 0.1 mM acetylcoenzyme A, 0.5 mg/ml liver cytosol, and 500 μM sulfamethazine. The reaction was initiated by the addition of acetylcoenzyme A and was then incubated for 60 min at 37°C. The reaction was terminated by adding 20 μl of ice-cold 70% perchloric acid. The product formation was determined using HPLC with a C18 5-μm analytical column (Mightysil, 4.6 × 150 mm; Kanto Kagaku, Tokyo, Japan). The mobile phase was acetonitrile/20 mM sodium acetate (pH 4.0) (5: 95, v/v). The flow rate was 1.0 ml/min and the column temperature was 35°C. The eluent was monitored at 254 nm with a Uni-3. Sulfamethazine N-acetyltransferase activity was quantified using a standard curve of sulfamethazine because we could not obtain authentic N-acetylsulfamethazine. The retention time of N-acetylsulfamethazine was confirmed using the incubation product of recombinant human NAT2 expressed in baculovirus-infected insect cells (BD Gentest) and sulfamethazine. The final concentration of the solvent in the incubation mixture was <1% except for estrone 3-sulfotransferase activity (2%).
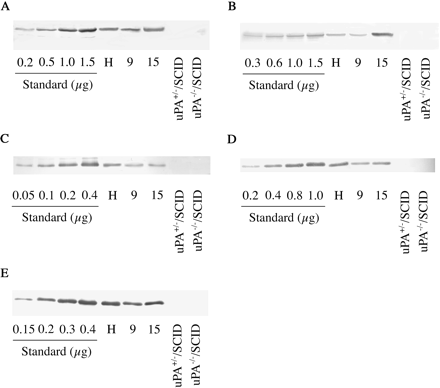
Selectivity of antibodies to human phase II enzymes on immunoblot analysis. Immunoblot analyses of microsomes and cytosol from pooled human liver (H), chimeric mice 9 and 15, and a control uPA+/–/SCID and a control uPA–/–/SCID mouse were performed using human UGT1A1 antibodies (A), human UGT2B7 antibodies (B), human SULT1E1 antibodies (C), human SULT2A1 antibodies (D), and human GSTA1 antibodies (E). A, the recombinant human UGT1A1 from BD Gentest (0.2, 0.5, 1.0, and 1.5 μg) was used as a standard. B, the recombinant human UGT2B7 from BD Gentest (0.3, 0.6, 1.0, and 1.5 μg) was used as a standard. C, the recombinant human SULT1E1 from Oxford Biomedical Research (0.05, 0.1, 0.2, and 0.4 μg) was used as a standard. D, the recombinant human SULT2A1 from Oxford Biomedical Research (0.2, 0.4, 0.8, and 1.0 μg) was used as a standard. E, the recombinant human GSTA1 from Oxford Biomedical Research (0.15, 0.2, 0.3, and 0.4 μg) was used as a standard.
DNA Sequencing. To confirm the NAT2 genotypes, DNA sequencing analysis was performed. The genomic DNA of the liver from a donor A chimeric mouse was extracted as described previously (Katoh et al., 2004). The genomic DNA was amplified using the primers (sense primer: 5′-GTCACACGAGGAAATCAAATGC-3′, antisense primer: 5′-GTTTTCTAGCATGAATCACTCTGC-3′) by PCR, and the entire coding region of NAT2 could be amplified. Then, the PCR product was subjected to DNA sequencing using a Thermo Sequenase Cy5.5 Dye Terminator Cycle Sequencing kit (Amersham Biosciences UK, Ltd., Little Chalfont, Buckinghamshire, UK). DNA sequences were analyzed on a Long-Read Tower DNA sequencer (Amersham Biosciences UK, Ltd.).
Results
Chimeric Mice Used in This Study. Nine chimeric mice generated using hepatocytes from donor A and five chimeric mice generated using those from donor B were used in the present study (Table 1).
Selectivity of Antibodies to Human Phase II Enzymes in Immunoblot Analysis. The selectivities of human antibodies were investigated in the sample from a chimeric mouse, a control uPA+/–/SCID mouse, and a control uPA–/–/SCID mouse (Fig. 1). The maximal amounts of protein not to cross-react with the autologous murine proteins using control murine liver microsomes and cytosol were as follows: UGT1A1, 40 μg; UGT2B7, 10 μg; SULT1E1, 40 μg; SULT2A1, 5 μg; and GSTA1, 5 μg. Human UGT1A1, UGT2B7, SULT1E1, and SULT2A1 antibodies, commercially available, were raised against a peptide of human UGT1A1, UGT2B7, SULT1E1, and SULT2A1, respectively. The antibodies used in the present study did not cross-react with control murine liver microsomes and cytosol in these experimental conditions.
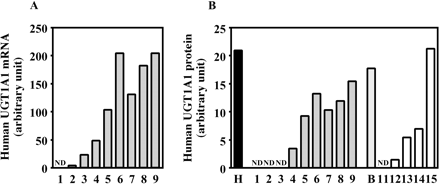
Human UGT1A1 expression in the chimeric mice. The expression of human UGT1A1 mRNA (A) and the expression of human UGT1A1 protein (B) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations. The sample numbers are described in Table 1. H, HLM; B, donor B; ND, not detected.
Human UGT1A9 expression in the chimeric mice. The expression of human UGT1A9 mRNA was determined as described under Materials and Methods. Each column represents the mean of duplicate determinations. The sample numbers are described in Table 1. ND, not detected.
Expression of Human UGT1A1 in Chimeric Mice. The expression of human UGT1A1 mRNA and human UGT1A1 protein was increased in a hAlb concentration-dependent manner (Fig. 2). The r values were 0.95 and 0.95 between mRNA or protein and a hAlb concentration in the donor A chimeric mice, respectively, and was 0.98 between protein and a hAlb concentration in the donor B chimeric mice.
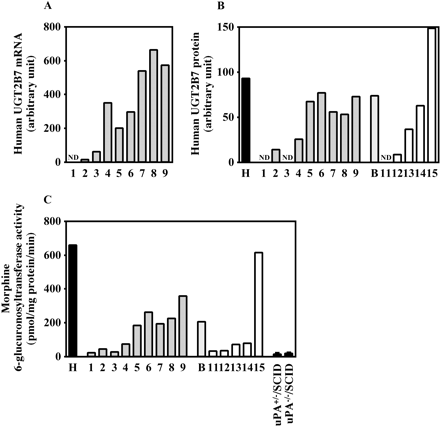
Human UGT2B7 expression in the chimeric mice. The expression of human UGT2B7 mRNA (A), the expression of human UGT2B7 protein (B), and morphine 6-glucuronosyltransferase activity (C) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations except in uPA+/–/SCID mice and uPA–/–/SCID mice. The columns of uPA+/–/SCID mice and uPA–/–/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 1. H, HLM; B, donor B; ND, not detected.
Expression of Human UGT1A9 in Chimeric Mice. The mRNA expression of human UGT1A9 is shown in Fig. 3. The expression of human UGT1A9 mRNA was increased in a hAlb concentration-dependent manner (r = 0.79).
Expression of Human UGT2B7 in Chimeric Mice. The human UGT2B7 mRNA and human UGT2B7 protein expression, and morphine 6-glucuronosyltransferase activity were increased in a hAlb concentration-dependent manner (Fig. 4). The r values were 0.93, 0.85, and 0.93 between mRNA, protein, or morphine 6-glucuronosyltransferase activity and a hAlb concentration, respectively, in the donor A chimeric mice and were 0.99 and 0.93 between protein or morphine 6-glucuronosyltransferase activity and a hAlb concentration, respectively, in the donor B chimeric mice. Morphine 6-glucuronosyltransferase activity in HLM (657.4 pmol/mg protein/min) was 41.3- and 34.8-fold higher than that in uPA+/–/SCID mice (15.9 pmol/mg protein/min) and uPA–/–/SCID mice (18.9 pmol/mg protein/min), respectively. This activity in microsomes from donor B (204.7 pmol/mg protein/min) was 31% of that in HLM.
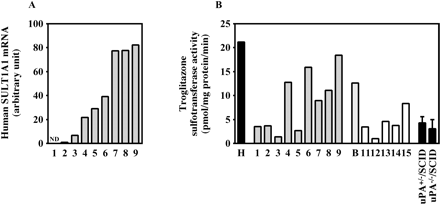
Expression of Human SULT1A1 in Chimeric Mice. The expression of human SULT1A1 mRNA and troglitazone sulfotransferase activity were increased in a hAlb concentration-dependent manner (Fig. 5). The r values were 0.97 and 0.71 between mRNA or troglitazone sulfotransferase activity and the hAlb concentration in the donor A chimeric mice, respectively, and was 0.82 between the troglitazone sulfotransferase activity and the hAlb concentration in the donor B chimeric mice. The troglitazone sulfotransferase activity in HLC (21.2 pmol/mg protein/min) was approximately 4.9- and 6.8-fold higher than that in uPA+/–/SCID mice (4.3 pmol/mg protein/min) and uPA–/–/SCID mice (3.1 pmol/mg protein/min), respectively. This activity in cytosol from donor B (12.6 pmol/mg protein/min) was 59% of that in HLC.
Human SULT1A1 expression in the chimeric mice. The expression of human SULT1A1 mRNA (A) and troglitazone sulfotransferase activity (B) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations except in uPA+/–/SCID mice and uPA–/–/SCID mice. The columns of uPA+/–/SCID mice and uPA–/–/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 1. H, HLC; B, donor B; ND, not detected.
Expression of Human SULT1B1 in Chimeric Mice. The mRNA expression of human SULT1B1 is shown in Fig. 6. The expression of human SULT1B1 mRNA was increased in a hAlb concentration-dependent manner (r = 0.71).
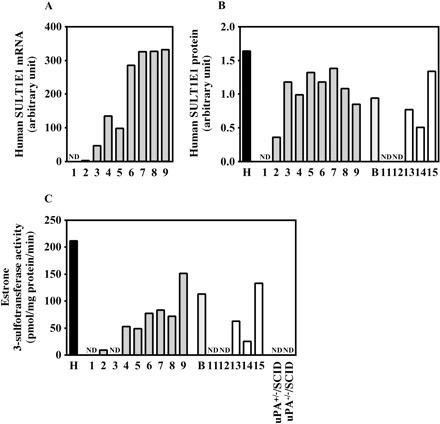
Expression of Human SULT1E1 in Chimeric Mice. The human SULT1E1 mRNA and human SULT1E1 protein expression, and estrone 3-sulfotransferase activity were increased in a hAlb concentration-dependent manner (Fig. 7). The r values were 0.96, 0.54, and 0.89 between mRNA, protein, or estrone 3-sulfotransferase activity and the hAlb concentration, respectively, in the donor A chimeric mice and were 0.90 and 0.90 between protein or estrone 3-sulfotransferase activity and the hAlb concentration, respectively, in the donor B chimeric mice. The estrone 3-sulfotransferase activity could not be detected in uPA+/–/SCID mice and uPA–/–/SCID mice in this experimental condition. This activity in cytosol from donor B (113 fmol/mg protein/min) was 53% of that in HLC (212 fmol/mg protein/min).
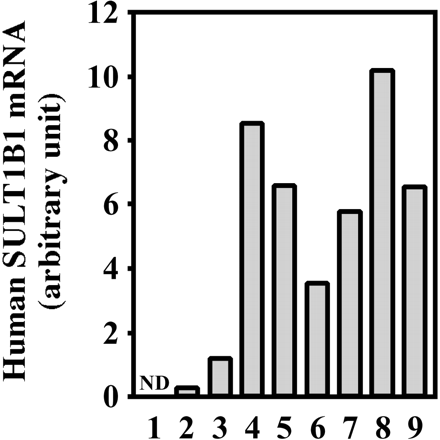
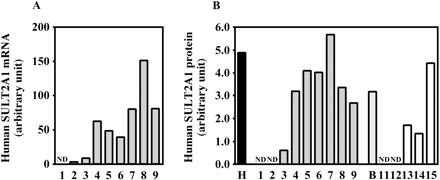
Expression of Human SULT2A1 in Chimeric Mice. The expression of human SULT2A1 mRNA and human SULT2A1 protein was increased in a hAlb concentration-dependent manner (Fig. 8). The r values were 0.85 and 0.72 between mRNA or protein and the hAlb concentration in the donor A chimeric mice, respectively, and was 0.95 between protein and the hAlb concentration in the donor B chimeric mice.
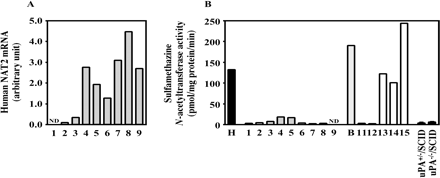
Expression of Human NAT2 in Chimeric Mice. The expression of human NAT2 mRNA and sulfamethazine N-acetyltransferase activity are shown in Fig. 9. The expression of human NAT2 mRNA was increased in a hAlb concentration-dependent manner (r = 0.82). In the case of the donor A chimeric mice, the sulfamethazine N-acetyltransferase activity appeared very low, although high activity was observed in the donor B chimeric mice. The sulfamethazine N-acetyltransferase activity in HLC (132.0 pmol/mg protein/min) was approximately 33.0- and 22.8-fold higher than in uPA+/–/SCID mice (4.0 pmol/mg protein/min) and uPA–/–/SCID mice (5.8 pmol/mg protein/min), respectively. This activity in cytosol from donor B (190.7 pmol/mg protein/min) was 144% of that in HLC.
Sequencing of NAT2 Gene. The entire coding region of NAT2 from a donor A chimeric mouse was analyzed. Two point mutations, C282T and G590A, were detected as heterozygotes.
Expression of Human GSTA1 in Chimeric Mice. The expression of human GSTA1 mRNA and human GSTA1 protein was increased in a hAlb concentration-dependent manner (Fig. 10). The r values were 0.84 and 0.88 between mRNA or protein and the hAlb concentration in the donor A chimeric mice, respectively, and was 0.90 between protein and the hAlb concentration in the donor B chimeric mice.
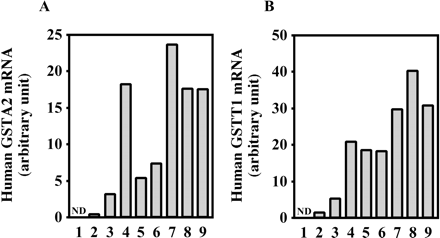
Expression of Human GSTA2 and Human GSTT1 in Chimeric Mice. The mRNA expression of human GSTA2 and human GSTT1 is shown in Fig. 11. The expression of human GSTA2 mRNA and human GSTT1 mRNA was increased in a hAlb concentration-dependent manner. The r values were 0.73 and 0.93 between the mRNA and the hAlb concentration, respectively, in the donor A chimeric mice.
Discussion
Endogenous substrates, xenobiotics including drugs, and/or the phase I metabolites are generally conjugated, which has been defined as a phase II reaction. Conjugation is known to occur in various reactions such as glucuronidation by UGT, sulfation by SULT, acetylation by NAT, and glutathione conjugation by GST. In drug development, preselection in terms of P450 metabolism has led to an increase in the relative involvement of phase II enzymes in the metabolic clearance of drugs (Fisher et al., 2001). One of the most important phase II reactions would be glucuronidation, and the glucuronides are more easily excreted into urine and bile than the parent compounds. Genetic polymorphisms of some UGT isoforms, especially UGT1A1, have been reported. Many mutated alleles of UGT1A1 have been identified and some mutations leading to decreases in the enzyme activity accounted for Crigler-Najjar syndrome and Gilbert's syndrome (Tukey and Strassburg, 2000). The expression of human UGT1A1 mRNA and UGT1A1 protein tends to correlate with the hAlb concentrations. The expression of human UGT in the chimeric mice could be monitored by the specific enzyme activity, catalyzed by human UGT but not by murine UGT. Since there are no specific substrates for human UGT1A1, we could not measure the enzyme activity of human UGT1A1 in the chimeric mice.
Human SULT1B1 expression in the chimeric mice. The expression of human SULT1B1 mRNA was determined as described under Materials and Methods. Each column represents the mean of duplicate determinations. The sample numbers are described in Table 1. ND, not detected.
Human SULT1E1 expression in the chimeric mice. The expression of human SULT1E1 mRNA (A), the expression of human SULT1E1 protein (B), and estrone 3-sulfotransferase activity (C) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations except in uPA+/–/SCID mice and uPA–/–/SCID mice. The sample numbers are described in Table 1. H, HLC; B, donor B; ND, not detected.
Williams et al. (2004) reported that one of the most important UGT isoforms was UGT2B7. The expression of human UGT2B7 mRNA and protein also increased in a hAlb concentration-dependent manner. Morphine is conjugated to 3-glucuronide and 6-glucuronide by UGT2B7. The ratios of UGT activity toward 3-glucuronidation and 6-glucuronidation in liver microsomes of mice, rats, guinea pigs, and rabbits were approximately 300:1, 90:1, 4:1, and 40:1, respectively, possibly indicating that species differences in the morphine glucuronosyltransferase activity exist (Kuo et al., 1991). In the present study, the morphine 6-glucuronosyltransferase activity in humans was approximately 36-fold higher than that in mice, suggesting that it would be appropriate to distinguish human UGT from murine UGT. The UGT2B7 protein and morphine 6-glucuronosyltransferase activity in chimeric mouse 15 were approximately 2.6- and 3.0-fold higher, respectively, than that in donor B. Human UGT2B7 might express easily in chimeric mouse 5 with high catalytic activity, but the reason remains to be clarified.
Sulfation is also an important metabolic reaction that results in detoxification or bioactivation. In human liver, the major cytosolic SULT isoform is likely to be SULT1A1 (Coughtrie and Fisher, 2003). Because specific anti-human SULT1A1 antibodies are not commercially available, we could not quantify the expression levels of human SULT1A1 protein. However, the expression levels of SULT1A1 mRNA were correlated with the hAlb concentrations in the chimeric mice. Troglitazone sulfate accounted for approximately 70% of the metabolites detected in human plasma (Shibata et al., 1993; Loi et al., 1997). Human SULT1A1 was shown to be mainly responsible for the sulfation of troglitazone in human liver (Honma et al., 2002) and showed higher activity toward troglitazone than murine SULT1A isoforms (Honma et al., 2001). In this experimental condition, the troglitazone sulfotransferase activity in humans was higher than that in mice. However, some chimeric mice with low hAlb concentrations exhibited similar activities compared with the control mice. It is surmised that the livers of those mice with low hAlb concentrations were reproduced by functional murine hepatocytes. In the comparison between the donor A and donor B chimeric mice with high hAlb concentrations, this enzyme activity in the donor A chimeric mouse was higher than that in the donor B chimeric mouse, suggesting that this phenomenon was caused by the interindividual variability of the donors. Using a more specific substrate for human, the differences of SULT1A1 enzyme activity between humans and mice would be significant, but we could not find it out. SULT1E1 is known to be a typical estrogen sulfotransferase. In this experimental condition, neither the uPA+/–/SCID nor the uPA–/–/SCID mice exhibited the sulfation of estrone, indicating that this would be a marker activity to evaluate the human SULT in chimeric mice. The expressions of SULT1E1 and SULT2A1 protein in chimeric mice 8 and 9 were low in comparison with those in chimeric mouse 7. Those two chimeric mice may cause the weak correlation between SULT1E1 or SULT2A1 protein and a hAlb concentration as well as between SULT1E1 protein and enzyme activity. It will be necessary to clarify the reason in the near future.
Human SULT2A1 expression in the chimeric mice. The expressions of human SULT2A1 mRNA (A) and human SULT2A1 protein (B) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations. The sample numbers are described in Table 1. H, HLC; B, donor B; ND, not detected.
Human NAT2 expression in the chimeric mice. The expression of human NAT2 mRNA (A) and sulfamethazine N-acetyltransferase activity (B) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations except in uPA+/–/SCID mice and uPA–/–/SCID mice. The columns of uPA+/–/SCID mice and uPA–/–/SCID mice represent the mean ± S.D. (n = 3). The sample numbers are described in Table 1. H, HLC; B, donor B; ND, not detected.
NAT carries out the acetylation of arylamines and arylhydrazines including drugs and environmental carcinogens. In humans, NAT2 is predominantly expressed in liver, whereas, on the other hand, NAT1 is mainly expressed in extrahepatic tissues (Levy and Weber, 2002). Interindividual variations in NAT functions are known and have been classified according to isoniazid metabolism, which is a typical substrate for NAT2 in humans. Such variations are mainly caused by genetic polymorphisms of the NAT2 gene, and many alleles have been identified. The frequency of peripheral neuropathy, which is the main adverse reaction of isoniazid, in a slow acetylator phenotype was reported to be higher than that in a rapid acetylator phenotype, and there was a negative correlation between the enzyme activity and the frequency of such adverse reactions (Hughes et al., 1954). Therefore, determining whether a drug candidate is a substrate of NAT2 is important. The expression levels of human NAT2 mRNA are correlated with the hAlb concentration. Since anti-human NAT2 antibodies are not commercially available, we could not evaluate NAT2 protein. Sulfamethazine is a typical substrate for human NAT2 (Grant et al., 1989) and is not metabolized by murine NAT (Martell et al., 1992). In the present study, the sulfamethazine N-acetyltransferase activity in humans was higher than that in mice, indicating that it is a suitable enzyme activity to evaluate the human NAT expression. The donor B chimeric mouse with a high hAlb concentration exhibited activity similar to that of donor B. However, these activities in all donor A chimeric mice were very low. These results could be explained by the retained genetic polymorphism of NAT2 in the chimeric mice. The frequency of the slow acetylator phenotype has been reported to be approximately 40 to 60% in whites and to be 10% in Japanese (Ellard, 1976; Cascorbi et al., 1995; Brocvielle et al., 2003). Many human NAT2 alleles have been identified, and most slow acetylators in whites were genotyped (but not conclusively) as homozygotes of NAT2*5 and NAT2*6 or NAT2*5/NAT2*6, NAT2*5/NAT2*7, NAT2*5/NAT2*13, NAT2*6/NAT2*7, and NAT2*6/NAT2*/13 (Cascorbi et al., 1995). Bolt et al. (2005) reported that the NAT2*13 allele was irrelevant as a determinant of the rapid acetylator phenotype, although the amino acids were not changed by this mutation. These NAT2*5, NAT2*6, NAT2*7, and NAT2*13 alleles exhibited the nucleotide changes of T341C, G590A, G857A, and C282T, respectively. As a result of the sequencing of NAT2, donor A was genotyped as NAT2*6/NAT2*13.
Human GSTA1 expression in the chimeric mice. The expression of human GSTA1 mRNA (A) and the expression of human GSTA1 protein (B) were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations. The sample numbers are described in Table 1. H, HLC; B, donor B; ND, not detected.
Generally, GST plays an important role in the detoxification of various xenobiotics. GST is involved not only in the metabolism of xenobiotics but also in the biosynthesis of leukotrienes and prostaglandins. In human liver, GST isoforms such as GSTA1, GSTA2, GSTM1, GSTT1, and GSTT2 are expressed. Recently, a strong correlation with the transaminase elevation by troglitazone was observed in the combined GSTT1 and GSTM1 null genotype (Watanabe et al., 2003). Also, these null genotypes increased the susceptibility to tacrine hepatotoxicity (Simon et al., 2000). Therefore, it is important to investigate whether a drug is catalyzed by a GST isoform to reduce the toxicity. GST antibodies specific for human isoforms expressed in liver except GSTA1 were not available, and the enzyme activities specific for human GST could not be found. The expression levels of human GSTA1 protein increased in a hAlb concentration-dependent manner with a high correlation. The human GSTM1 mRNA in donor A chimeric mice could not be detected by TaqMan RT-PCR (data not shown), suggesting that the GSTM1 gene was deleted in donor A.
Human GSTA2 and GSTT1 expression in the chimeric mice. The expression of human GSTA2 (A) and GSTT1 (B) mRNA were determined as described under Materials and Methods. Each column represents the mean of duplicate determinations. The sample numbers are described in Table 1. ND, not detected.
Due to the lack of specificities of antibodies and enzyme activities, the investigation of phase II enzymes in the chimeric mice was very difficult, but the results described above clarified the expression of human UGT, SULT, NAT, and GST in the chimeric mice.
In the present study, 11- to 14-week-old chimeric mice were used. Further study may be needed to confirm the long-term stability of the expression of human drug-metabolizing enzymes and humanized phenotype in the elder chimeric mice. Due to the limited supply of hepatocytes from one donor, it is sometimes difficult to perform a study using chimeric mice generated from the same donor. In the previous and present studies, we focused on drug-metabolizing enzymes. Using the chimeric mice generated from various donors, further study to clarify the effect of genetics, diet, age, and gender between human donors will be performed in the near future.
In conclusion, the human phase II enzymes in the chimeric mice expressed and possessed the enzyme activity. In our previous study, the livers of the chimeric mice expressed human P450s and exhibited enzyme activities similar to those in humans (Katoh et al., 2004). That the livers of the chimeric mice expressed both human phase I and phase II enzymes is of great importance. Furthermore, in vivo studies using chimeric mice would be useful because we could evaluate the metabolism of drugs catalyzed by both phase I and phase II enzymes in vivo. The results of the present study provide valuable information concerning the chimeric mice with humanized liver. Studies on the drug metabolism of chimeric mice are still in progress.
Acknowledgments
We acknowledge Brent Bell for reviewing the manuscript.
Footnotes
-
This work was supported by a Research on Advanced Medical Technology, Health, and Labor Sciences Research Grant from the Ministry of Health, Labor, and Welfare of Japan.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.005157.
-
ABBREVIATIONS: P450, cytochrome P450 enzyme; uPA, urokinase-type plasminogen activator; SCID, severe combined immunodeficient; hAlb, human albumin; HLM, pooled human liver microsomes; HLC, pooled human liver cytosol; UGT, UDP-glucuronosyltransferase; SULT, sulfotransferase; NAT, N-acetyltransferase; GST, glutathione S-transferase; HPLC, high-performance liquid chromatography; RT-PCR, reverse transcription-polymerase chain reaction; PAPS, adenosine 3′-phosphate 5′-phosphosulfate; RI, replacement index.
- Received April 12, 2005.
- Accepted June 1, 2005.
- The American Society for Pharmacology and Experimental Therapeutics