Abstract
Cytochrome P450 is a family of enzymes that catalyze reactions involved in the metabolism of drugs and other xenobiotics. These enzymes are therefore important in pharmacologic and toxicologic studies, and information on their abundances is of value in the process of scaling in vitro data to in vivo metabolic parameters. A meta-analysis was applied to data on the abundance of human hepatic cytochrome P450 enzymes in Caucasian adult livers (50 studies). Despite variations in the methods used to measure the abundance of enzymes, agreement between the studies in 26 different laboratories was generally good. Nonetheless, some heterogeneity was detected (Higgins and Thompson heterogeneity test). More importantly, large interindividual variability was observed in the collated data. Positive correlations between the expression levels of some cytochrome P450 enzymes were found in the abundance data, including the following pairs: CYP3A4/CYP3A5*1/*3 (Rs = 0.70, P < 0.0001, n = 52), CYP3A4/CYP2C8 (Rs = 0.68, P < 0.0001, n = 134), CYP3A4/CYP2C9 (Rs = 0.55, P < 0.0001, n = 71), and CYP2C8/CYP2C9 (Rs = 0.55, P < 0.0001, n = 99). These correlations can be used to demonstrate common genetic transcriptional mechanisms.
Introduction
Cytochrome P450 enzymes constitute the main family of enzymes responsible for phase I metabolism reactions (Al Omari and Murry, 2007). The survey by Wienkers and Heath (2005) indicated the involvement of these enzymes in the metabolic clearance of nearly 75% of the 200 most prescribed therapeutic drugs in the United States in 2002. A report by Zanger et al. (2014) described the contribution of each drug-metabolizing cytochrome P450 enzyme to drug metabolism pathways, as well as the various genetic and nongenetic factors that affect variability in enzymatic activity and, therefore, the clinical outcome of therapy.
Many studies have attempted to quantify the abundance of cytochrome P450 enzymes and to describe the genetic and epigenetic factors that affect the expression of these enzymes. As indicated by Proctor et al. (2004), abundance data of cytochrome P450 can be used for extrapolation from in vitro data to in vivo pharmacokinetic parameters, particularly when constructing the population variability is of interest. Hence, these data can be used for simulation experiments involving virtual populations for the pharmacologic and toxicologic assessment of drugs (Rostami-Hodjegan and Tucker, 2007). Correlations of expression between enzymes have been reported in the literature (Jover et al., 2009), and although in the current framework of Monte-Carlo simulations for creating virtual populations (Rostami-Hodjegan and Tucker, 2007) these correlations are not considered, such correlations, if established, can assist in producing more reliable scenarios.
Many efforts have been made to quantify cytochrome P450 enzymes, and some of these studies attempted to assess correlations of expression. These quantitative data were obtained using a variety of methods [Western blotting, enzyme-linked immunosorbent assay (ELISA), or liquid chromatography in conjunction with mass spectrometry (LC-MS)] and using different numbers of subjects from different age groups and ethnic backgrounds.
Meta-analysis is a process of combining different studies to obtain an overall figure for a measured variable, detect the degree of heterogeneity between the individual studies, and describe inconsistency and its possible sources (Sánchez-Meca and Martín-Martínez, 1997). With the growing number of studies that quantify pharmacokinetically relevant enzymes, there is a pressing need for a meta-analysis of these data to identify the weighted mean abundance levels of the enzymes, the degree of variability in these abundance data, and the degree of heterogeneity between these studies. The most recent meta-analysis of abundance data of drug-metabolizing cytochrome P450 enzymes, published in 2004, analyzed data from 19 studies and was a comprehensive and influential analysis, even though it did not distinguish between CYP3A isoforms (Rowland-Yeo et al., 2004) (see Supplemental Fig. 1). Since 2004, an additional 31 important studies of cytochrome P450 abundance have been published; for this reason, we now present a meta-analysis of the literature on cytochrome P450 abundance published up to the beginning of 2014 and provide correlations of expression where data are available. This meta-analysis will assist different groups who work with physiologically based pharmacokinetic models to incorporate more representative values for the system parameters related to the expression levels of cytochrome P450 enzymes.
Materials and Methods
Collection of Data.
Two electronic databases, Medline (http://www.nlm.nih.gov/bsd/pmresources.html) and Web of Knowledge (http://wok.mimas.ac.uk/) (between the years 1980 and 2014), were searched for relevant literature on the abundance of enzymes by using appropriate key words (hepatic/liver cytochrome P450 abundance, hepatic/liver CYP abundance, correlation of expression). Other key words were also used (e.g., quantity, concentration, content, quantification, measurement) instead of abundance to widen the search scope. Resources cited by the collected articles were also inspected to locate additional literature that could be used. A set of predefined criteria were used for inclusion of identified studies. The selected studies were those conducted on individual liver microsomal samples (rather than pooled samples or cell lines), where absolute protein abundance was measured in Caucasian adults. In addition, studies quoting abundance data in arbitrary, relative, or nonstandard units (other than pmol mg−1) were excluded. Finally, studies that quantified mRNA levels or enzyme activities only were also excluded. The sources of data were identified to ensure that none of the data used were duplicated in the analysis. Where data were not quoted and graphs contained data points, GetData Graph Digitizer version 2.25 (available at: http://www.getdata-graph-digitizer.com/) was used to obtain abundance values.
Calculation of Weighted Means and Coefficients of Variation.
For each enzyme, abundance values were tested for heterogeneity and normality of distribution. The Higgins and Thompson heterogeneity test (Cochran, 1954; Higgins and Thompson, 2002; Higgins et al., 2003) determines whether the 50 studies are consistent with one another, whereas the normality test determines whether the combined data are normally distributed. The normality test was performed according to the method of Kolmogorov and Smirnov.
The data from the individual studies were combined. The weighted means (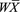 ) and the weighted coefficients of variation (WCVs) of the abundance of the different enzymes from the collected studies were calculated using eqs. 1 and 2 (Armitage et al., 2001), respectively:
) and the weighted coefficients of variation (WCVs) of the abundance of the different enzymes from the collected studies were calculated using eqs. 1 and 2 (Armitage et al., 2001), respectively: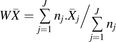 (1)
(1)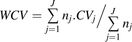 (2)where (
(2)where ( ) and WCV represent the weighted mean and weighted coefficient of variation, respectively. Subscript j indicates the study, nj the number of samples in study j, and
) and WCV represent the weighted mean and weighted coefficient of variation, respectively. Subscript j indicates the study, nj the number of samples in study j, and 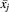 the mean abundance of a particular enzyme in study j.
the mean abundance of a particular enzyme in study j.
Assessment of Heterogeneity between Studies.
To assess the homogeneity between the means and coefficients of variation of individual studies and the overall mean and variability of the collated data, eqs. 3, 4, and 5 were used: (3)
(3)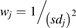 (4)
(4)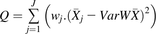 (5)where wj is the weight of study j expressed as the variance and calculated using eq. 4 (the inverse of standard deviation, sdj, squared), (
(5)where wj is the weight of study j expressed as the variance and calculated using eq. 4 (the inverse of standard deviation, sdj, squared), (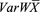 ) is the variance in the weighted mean of the data from all the studies. Q is the coefficient of heterogeneity (eq. 5) of the collated data [Cochran’s Q test (Cochran, 1954)] expressed as the collective weighted squared differences between the mean of each study and the variance in the weighted mean. A higher value of Q indicates greater heterogeneity.
) is the variance in the weighted mean of the data from all the studies. Q is the coefficient of heterogeneity (eq. 5) of the collated data [Cochran’s Q test (Cochran, 1954)] expressed as the collective weighted squared differences between the mean of each study and the variance in the weighted mean. A higher value of Q indicates greater heterogeneity.
The degree of heterogeneity can be assessed using the I2 index (eq. 6) proposed by Higgins and Thompson (2002). This index provides a percentage of overall heterogeneity that can be interpreted as proposed by Higgins et al. (2003) as follows: around 0%, no heterogeneity, around 25%, low heterogeneity, around 50%, moderate heterogeneity, and around 75%, high heterogeneity: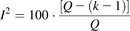 (6)where I2 is the index of heterogeneity, Q is Cochran’s heterogeneity coefficient, and (k – 1) is the number of degrees of freedom defined as the number of studies, k, minus one. Where I2 is negative, it is set to zero. The probability, P, of the test can be quoted by comparing the Q value with a χ2 distribution with the same number of degrees of freedom (Higgins et al., 2003).
(6)where I2 is the index of heterogeneity, Q is Cochran’s heterogeneity coefficient, and (k – 1) is the number of degrees of freedom defined as the number of studies, k, minus one. Where I2 is negative, it is set to zero. The probability, P, of the test can be quoted by comparing the Q value with a χ2 distribution with the same number of degrees of freedom (Higgins et al., 2003).
Assessing Correlations between the Abundances of Drug-Metabolizing Enzymes.
The Spearman rank test (Armitage et al., 2001) was used to assess correlations of the expression levels of the enzymes. Before correlation calculations, collated data were normalized using mean values of reference studies (eq. 7).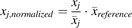 (7)where
(7)where 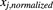 is the normalized abundance value of the abundance measurement x in study j,
is the normalized abundance value of the abundance measurement x in study j,  is the mean abundance value of study j, and
is the mean abundance value of study j, and 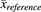 is the mean abundance of the same enzyme in the reference study used for the normalization process. The reference abundance values used for normalization were those obtained in this meta-analysis (Table 1).
is the mean abundance of the same enzyme in the reference study used for the normalization process. The reference abundance values used for normalization were those obtained in this meta-analysis (Table 1).
The weighted means, coefficients of variation (CV), ranges, and heterogeneity analysis of the analyzed hepatic cytochrome P450 enzyme abundance data from 50 studies
Calculations of the Spearman correlation coefficients, Rs, and probabilities, P, were carried using a t-Student distribution. A Bonferroni correction was used to define the P value for statistical significance for the number of tests applied on each enzyme (a maximum of 14 tests for each enzyme).
Assessment of Gender- and Age-Related Differences in Expression.
Differences between expression levels in Caucasian adult liver cytochrome P450 enzymes in male and female subjects and in different age groups were investigated where data were available. Gender-related differences were investigated using the Mann-Whitney rank order U test, and correlation of expression with age was examined using the Spearman rank correlation test.
Results
Studies Used for the Meta-analysis.
The number of studies used in the meta-analysis was 50 of 94 studies collected using the search strategy. Several criteria were used to select the studies to be used for the meta-analysis. Studies were excluded where the samples were not individual liver microsomal samples (either recombinantly expressed systems, cell lines, or pooled samples; 13 studies), where enzymatic activity or mRNA levels of expression were the only quantification data (19 studies) and where all the study subjects were from a non-Caucasian ethnic group (six studies) or from a pediatric age group (four studies). Additionally, studies quoting abundance data in arbitrary, relative, or nonstandard units (other than pmol mg−1) were also excluded (seven studies). Some studies were excluded for more than one of these reasons.
The data in the selected studies for the meta-analysis were obtained in 26 different laboratories worldwide using two different types of experimental method for abundance measurement: immunoquantification (Western blotting or ELISA) and LC-MS. Most of the studies included in the meta-analysis used Western blotting (44 studies), whereas ELISA was used in three studies (Stresser and Kupfer, 1999; Snawder and Lipscomb, 2000; Barter et al., 2010) and LC-MS was used in five studies (Wang et al., 2008; Langenfeld et al., 2009; Seibert et al., 2009; Ohtsuki et al., 2012; Achour et al., 2014a). Wang et al. (2008); Langenfeld et al. (2009) used both immunoquantification and LC-MS. The range of enzymes quantified and the number of livers used in the studies were different for each enzyme (Table 1).
For correlation analysis, additional studies conducted on samples from non-Caucasian (Kawakami et al., 2011) and pediatric populations (Koukouritaki et al., 2004; Hines, 2007) were also included. Studies expressing abundance in relative units (Wrighton et al., 1987; Wrighton et al., 1993) were also used.
Meta-analysis of Cytochrome P450 Enzyme Abundance.
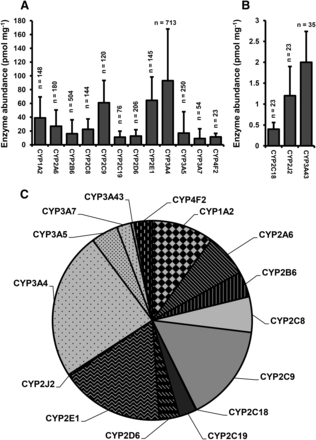
The data from available literature were collated and tested for normality and heterogeneity. Data did not show normal distribution. The data from different studies did not show extensive heterogeneity except for the CYP3A43 data set, which came from only two independent studies. Table 1 shows the results of the heterogeneity tests and the WXs and WCVs. Figure 1 shows the results of the meta-analysis for the 15 drug-metabolizing cytochrome P450 enzymes and a pie chart of the distribution of expression of these enzymes in hepatic tissue. The bar graph in Fig. 1 was divided into panels A and B to make it possible to visualize clearly the low abundance enzymes (CYP2C18, CYP2J2, and CYP3A43) using two different scales. Cytochrome P450 protein abundance pie charts from this meta-analysis and previous studies (Shimada et al., 1994; Rowland-Yeo et al., 2004) are shown in Supplemental Fig. 1.
Bar graph (A and B) and pie chart (C) of weighted mean abundances of cytochrome P450 enzymes in livers from adult Caucasians. Error bars represent weighted standard deviation values. n, the number of livers.
Correlation Analysis of the Abundance of Cytochrome P450 Enzymes.
Where more than one enzyme was quantified in the same samples, the data were used for correlation analysis. This analysis included data from other ethnic backgrounds and from pediatric samples. Since the data were not normally distributed, the Spearman rank correlation test was used after normalizing the collated data using mean values obtained in this meta-analysis (Table 1). Table 2 shows the correlation matrix obtained using the correlation analysis, and Fig. 2 shows examples of statistically significant weak and strong correlations.
Correlations matrix of the abundance data of 15 cytochrome P450 enzymes from 32 studies
Plots of examples of significant correlations of hepatic cytochrome P450 expression levels. Correlations between CYP2B6 and CYP3A4 (A) and between CYP2C9 and CYP2C19 (E) are weak, whereas correlations between CYP2C8 and CYP2C9 (B), CYP2C8 and CYP3A4 (C), and CYP2C9 and CYP3A4 (D) are strong. The strongest correlation was found between CYP3A4 and CYP3A5*1/*3 (F). Plots show normalized collated data.
Gender- and Age-Related Differences of Expression.
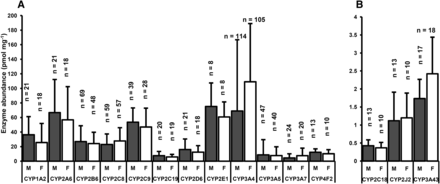
No statistically significant differences in cytochrome P450 abundance levels were found between male and female Caucasian subjects (Mann-Whitney U test, P > 0.05) for all data sets except CYP3A4, which was higher in livers from female subjects (n = 105) than in those from male subjects (n = 114) (Mann-Whitney U test, P < 0.0001) (Fig. 3). In addition, no correlation between hepatic levels of enzyme expression and age was found in Caucasian adults (Supplemental Fig. 2) except in the case of CYP2C9 (Spearman correlation test, P < 0.05, n = 60).
Expression of cytochrome P450 in male and female Caucasian subjects. No sex differences were detected (U-test, P > 0.05) in any cases, except between levels of CYP3A4 in male and female livers (U-test, P < 0.0001). F, female; M, male; n, number of subjects in each set.
Discussion
Abundance levels and activities of drug-metabolizing enzymes play major roles in determining the fate of drugs and other xenobiotics in the body. In addition, pharmacokinetic evaluation of a new therapeutic entity, in the process of drug development, has become increasingly reliant on the use of in vitro systems that are dependent on scaling factors, which include the abundance data of enzymes involved in metabolism pathways relevant to the particular drug (Barter et al., 2007; Rostami-Hodjegan and Tucker, 2007).
This study aimed to analyze the published literature on the abundance of cytochrome P450 enzymes in adult Caucasian livers. Using the defined criteria, 50 individual studies from 26 different laboratories were selected for analysis. The number of livers used to quantify each enzyme ranged from 23 to 713 livers, with the CYP3A4 data set having the highest number of livers (n = 713) with the largest number of studies (k = 31). Some heterogeneity was found between data from different studies, especially in the case of CYP3A43; however, in the absence of information on interlaboratory consistency of assays, it is difficult to assign these differences to heterogeneity between samples. Nevertheless, it was clear that when Western blotting was the method of quantification, significant differences were found between abundance levels when different standards were used. Heterogeneity also tended to become less noticeable between data sets generated using the same quantitative method (immunoquantification or LC-MS). Data sets collected from a small number of studies tended to show higher levels of heterogeneity (especially in the case of CYP3A43), which should be interpreted with caution as the decreased numbers of degrees of freedom in these cases significantly diminish the power of the heterogeneity test (Higgins and Thompson, 2002; Higgins et al., 2003). Other sources of heterogeneity may include different protocols of the same method (especially in the case of Western blotting) and low numbers of samples in some studies, which can cause the effect of outliers to be magnified.
The data obtained in this study can be used as scaling factors for in vitro-in vivo extrapolation of measurements from in vitro recombinant enzyme systems and in simulations of drug trials and pharmacokinetic experiments (Table 1) (Barter et al., 2007; Rostami-Hodjegan and Tucker, 2007). The meta-analysis data revealed large interindividual variation across cytochrome P450 enzymes (5- to 1300-fold difference), highlighting the importance of taking variability into account in the process of in vitro-in vivo extrapolation (Rostami-Hodjegan and Tucker, 2007). Another aspect that can be incorporated in the process of pharmacokinetic simulation and the building of virtual populations is the correlation of expression between pharmacokinetically relevant proteins (Table 2). The strongest correlation was seen between levels of CYP3A4 and CYP3A5*1/*3 genetic variant (Rs = 0.70, P < 0.0001, n = 52, nine studies). This correlation was not strong when the CYP3A5 genotype was ignored (Rs = 0.06, P = 0.37, n = 218), a consistent observation in the literature (Lin et al., 2002; Barter et al., 2010; Achour et al., 2014a). Strong and statistically significant correlations between cytochrome P450 enzymes identified also include the following pairs: CYP3A4/CYP2C8 (Rs = 0.68, P < 0.0001, n = 134), CYP3A4/CYP2C9 (Rs = 0.55, P < 0.0001, n = 71), CYP2C8/CYP2C9 (Rs = 0.55, P < 0.0001, n = 99), and CYP1A2/CYP2C9 (Rs = 0.56, P < 0.0001, n = 53). Although a relatively strong correlation between CYP2B6 and CYP3A4 expression levels was reported in individual studies in the literature (Rs > 0.5) (Mimura et al., 1993; Totah et al., 2008; Achour et al., 2014a), this correlation was weaker on analysis of all the collected data (Rs = 0.46, P < 0.0001, n = 124, five studies). Correlations of expression have also been reported at the mRNA level (Wortham et al., 2007), which further supports reports of common genetic regulation of the expression of cytochrome P450 enzymes (Jover et al., 2009).
Although some of the correlations demonstrated by this meta-analysis are strong, it is necessary to exercise caution in interpreting these relationships. All the correlations observed were positive, and although this would be expected [correlation arising from common regulatory receptors and pathways (Wortham et al., 2007; Jover et al., 2009)], there appears to be a background positive correlation between all the cytochrome P450 enzymes under study. The background correlation is a necessary artifact of measuring abundance levels in units of pmol mg−1. A Bonferroni correction of the P value made the test stricter and provided more confidence in the strong correlations observed. Recently, it has been proposed that abundance levels would be more usefully measured in units of micrograms per gram of tissue in the case of uridine 5′-glucuronosyltransferase (UGT) enzymes (Milne et al., 2011), and the findings reported here may lend support to this suggestion.
Aging in adults (18 years onward) seemed to correlate with an overall apparent decline in expression of cytochrome P450 enzymes; however, correlation between age and levels of cytochrome P450 was not statistically significant (Spearman rank test, P > 0.05; Supplemental Fig. 2) for all the data sets except one (CYP2C9). In adult subjects, age was previously reported to have minimal effect on the abundance and activity of cytochrome P450 enzymes per mass unit of liver (King et al., 2003; Wolbold et al., 2003; Galetin et al., 2004). However, analysis of reported metabolic ratios (which were constant with age) indicated a decrease in the liver metabolic activity mediated by cytochrome P450 enzymes that is paralleled by the decline in renal function with age (Rostami-Hodjegan et al., 1999). Two contributing factors to these seemingly contradictory observations (i.e., no change in abundance per unit mass with age and reduced total metabolic activity) can be liver shrinkage that occurs when body size decreases with age (Johnson et al., 2005) and the decrease in the amount of total microsomal protein per gram liver (Barter et al., 2008). Therefore, although the abundance levels of expressed cytochrome P450 enzymes seem not to be affected by aging to a significant extent, the literature suggests that overall activity may be affected. The results of a recent study by Polasek et al. (2013) are also supportive of this view, although the area requires further investigation.
Gender differences in expression of cytochrome P450 were not statistically significant except in the case of CYP3A4, in line with literature on CYP3A4 abundance (Wolbold et al., 2003). This finding is supported by reports of more efficient clearance of CYP3A substrates, such as nifedipine (Krecic-Shepard et al., 2000), verapamil (Wolbold et al., 2003), and cyclosporine (Kahan et al., 1986), in female subjects. CYP3A4 is highly inducible, and only one study (Wolbold et al., 2003) has distinguished between inducer-exposed and control subjects. In the controls, CYP3A4 levels were on average 2.6 times higher in female than in male subjects (n = 39), whereas in inducer-exposed patients, the levels were much closer (1.7-fold difference in the mean, n = 55). The headline numbers would suggest that gender might influence clinical practice in prescribing drugs metabolized by CYP3A4; the reality, however, is that there is overlap between males and females in CYP3A4 expression and that exposure to inducers is much more significant than gender.
It is interesting to compare the present work with the single study (Shimada et al., 1994) published in 1994 and with the first meta-analysis (Rowland-Yeo et al., 2004) published in 2004 (see Supplemental Fig. 1). When restricted to the enzymes detectable in 1994 (CYP1A2, 2A6, 2B6, 2C, 2D6, 2E1, 3A), the pie charts are very similar, with the most striking difference being the growth of the relative abundance of CYP2B6 at the expense of CYP3A. We attribute this difference to the cross-reactivity of antibodies (early antibodies to CYP3A4 were rather nonspecific). The major difference between this work and earlier work, however, is the sheer number of individual enzymes detected; 15 individual enzymes have now been quantified, allowing a much more detailed human liver “pie” to be created and complementing our recent UGT liver pie (Achour et al., 2014b).
We look forward to extending this work to describe the expression profiles of cytochrome P450 in different ethnic groups and in pediatric samples in addition to investigating correlations of expression between different proteins (enzymes and transporters) that govern the processes of metabolism, disposition, and elimination of drugs in different organs, including the liver, the intestine, and the kidney.
Acknowledgments
The authors thank Eleanor Savill for assistance in preparing the manuscript.
Authorship Contributions
Participated in research design: Achour, Barber, Rostami-Hodjegan.
Performed data analysis: Achour.
Wrote or contributed to the writing of the manuscript: Achour, Barber, Rostami-Hodjegan.
Footnotes
- Received April 30, 2014.
- Accepted May 30, 2014.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- ELISA
- enzyme-linked immunosorbent assay
- LC-MS
- liquid chromatography in conjunction with mass spectrometry
- P450
- cytochrome P450
- WCV
- weighted coefficient of variation
- WX
- weighted mean
- Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics