Abstract
Our recent study carried out after local injection of the serotonergic neurotoxin 5,7-dihydroxytryptamine into the arcuate nucleus (ARC) of the hypothalamus suggested a positive influence of the serotonergic innervation of the ARC on growth hormone (GH) secretion and GH-dependent expression of cytochrome P450. The aim of our present study was to determine the effect of the activation of the serotonin (5-HT)-type receptors, 5-HT1 or 5-HT2, in the ARC on the expression and activity of cytochrome P450 in the liver of male rats. The serotonergic agonist 5-carboxyamidotryptamine [(5-CT), a 5-HT1-type receptor agonist] or 2,5-dimethoxy-4-iodoamphetamine [(DOI), a 5-HT2-type receptor agonist] was injected into the ARC for 5 days. The activity and expression of cytochrome P450 isoenzymes and the levels of serum and pituitary hormones were estimated. DOI significantly increased the activity and expression (both mRNA and protein levels) of CYP2C11, CYP3A1/23, and CYP3A2, which positively correlated with an increase in the pituitary growth hormone–releasing hormone (GHRH) and serum GH level. The injection of 5-CT into the ARC did not affect the activity of liver P450 enzymes or hormone levels. The obtained results indicate that 5-HT2, but not the 5-HT1-type receptors in the ARC, are engaged in the positive neuroendocrine regulation of cytochrome P450, possibly by the stimulation of hypothalamic GHRH release and pituitary GH secretion, and an increase in the serum GH concentration. Further studies are going to identify which of the 5-HT2 receptor subtypes (5-HT2A, 5-HT2B, or 5-HT2C) is responsible for the observed neuroendocrine regulation of cytochrome P450.
Introduction
Our earlier studies conducted after damage to or stimulation of the brain serotonergic system by intracerebroventricular administration of the serotonergic neurotoxin 5,7-dihydroxytryptamine or 5-hydroxytryptophan, respectively, showed an important role of this brain system in the physiologic neuroendocrine regulation of cytochrome P450 expression and activity in the liver (Rysz et al., 2015, 2016a). Moreover, the results obtained using local intrahypothalamic injection of the serotonergic neurotoxin 5,7-dihydroxytryptamine showed the reverse effect of the serotonergic innervation of the paraventricular nucleus (PVN) and arcuate nucleus (ARC) of the hypothalamus on the regulation of the main male cytochrome P450 enzyme, CYP2C11, in the rat (Rysz et al., 2016b). The observed effect was negative in the case of the PVN and positive in the case of the ARC, and was accompanied by respective changes in growth hormone (GH) secretion. Recent studies carried out after repeated injection of selective serotonin (5-HT) receptor agonists, 5-HT1 and 5-HT2, have revealed that activation of the 5-HT1A receptor in the PVN negatively regulates liver cytochrome P450 expression and activity in the rat. The activation of the 5-HT1A receptor in the PVN produced an increase in somatostatin release and a decrease in growth hormone and corticosterone concentration in the blood, which led to a reduction in the hormone-dependent CYP2C11 and CYP3A1/23 expression and activity in the liver (Bromek et al., 2018).
It has been suggested that the stimulating effect of brain serotonin on GH secretion may proceed via 5-HT1 or 5-HT2 receptor-type stimulation, which increases the release of growth hormone–releasing hormone (GHRH) from the ARC (Murakami et al., 1986; Willoughby et al., 1987; Katz et al., 1996). Arcuate nuclei receive serotonergic innervation from the frontal raphe groups of serotonergic neurons such as the dorsal and median raphe nuclei (Gruber et al., 1987; Willoughby and Blessing, 1987). By acting on its receptors in the ARC, serotonin may affect the synthesis and release of GHRH, which in turn stimulates pituitary secretion of GH.
GH is the main physiologic regulator of liver CYP2C11 and one of the regulators of other cytochrome P450 enzymes, such as CYP3A enzymes in rat liver (Waxman and O’Connor, 2006; Waxman and Holloway, 2009; Wójcikowski and Daniel, 2011). Our previous studies showed that the ARC-mediated stimulation of pituitary GH secretion was strongly involved in the regulation of cytochrome P450 by brain monoaminergic neurotransmitters. Damage to one of the monoaminergic innervation pathways of the ARC (dopamine, noradrenaline, or serotonin) decreased plasma GH level and GH-stimulated cytochrome P450 expression and activity (Wójcikowski et al., 2007; Wójcikowski and Daniel, 2009; Bromek et al., 2013; Rysz et al., 2016b). However, a particular 5-HT receptor type/subtype of the ARC, which is engaged in the neuroendocrine regulation of liver cytochrome P450, has not been found to date.
Considering the possible presence of both 5-HT1 and 5-HT2 receptor types in the ARC (Steffens et al., 2008), the aim of this work was to identify the 5-HT receptor types located in the ARC (5-HT1 or 5-HT2) that are engaged in cytochrome P450 regulation in the liver. To achieve this goal we injected selective 5-HT1 and 5-HT2 receptor agonists in pharmacologically active doses into the ARC. GHRH produced therein is conveyed by neuronal transport to the median eminence, and then via portal circulation it is carried forward to the pituitary where it stimulates the secretion of GH (Bluet-Pajot et al., 1998; Wójcikowski and Daniel, 2011). Therefore, the levels of pituitary GHRH and serum GH as well as serum concentrations of other hormones that may directly regulate cytochrome P450 (corticosterone and thyroid hormones) were measured. At the same time, hormone-dependent expression and activity of liver cytochrome P450 enzymes were examined. The obtained results indicate a new neuroendocrine mechanism of liver cytochrome P450 regulation by the brain serotonergic system.
Materials and Methods
Animals.
Male Wistar Han rats weighing 280–300 g (Charles River Laboratories, Sulzfeld, Germany) were housed under standard laboratory conditions (12-hour light/12-hour dark cycle; temperature of 22 ± 2°C; room humidity of 55% ± 5%). The animals had free access to tap water and standard laboratory food; however, 18 hours before decapitation, they were deprived of food. All experiments were carried out in accordance with the 86/609/EEC Directive (Commission of the European Communities, 1986) and received approval from the Local Bioethics Commission at the Institute of Pharmacology of the Polish Academy of Sciences in Kraków.
Drugs and Chemicals.
The 5-HT1 receptor agonist 5-carboxyamidotryptamine (5-CT) maleate was obtained from Sigma (St. Louis, MO) and the 5-HT2 receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) hydrochloride was obtained from Tocris (Bristol, UK). Testosterone and its specific hydroxy metabolites were provided by Steraloids (Newport, KY). The following antibodies were used: polyclonal primary anti-rat CYP2C11 antibody from Abcam (Cambrige, UK), anti-rat CYP3A1/23 and CYP3A2 antibodies from Millipore (Temecula, CA), and polyclonal anti-rat β-actin antibody from Santa Cruz (Dallas, TX). The chemiluminescence reagents LumiGlo kit came from KPL (Gaithersburg, MD). Enzyme-linked immunosorbent assay (ELISA) kits for pituitary GHRH (Elabscience, Bethesda, MD), growth hormone and corticosterone (Demeditec, Kiel, Germany), and triiodothyronine (T3) and thyroxine (T4) (Cloud-Clone Corp., Katy, TX) were used. A mirVana kit, TaqMan assays, and the TaqMan Gene Expression Master Mix from Life Technologies (Carlsbad, CA), and a Transcriptor High-Fidelity cDNA synthesis kit from Roche Diagnostics (Indianapolis, IN) were used for RNA isolation and mRNA estimation. Ketamine hydrochloride and xylazine hydrochloride were produced by Biowet (Puławy, Poland).
Surgery and Local Injection of 5-CT or DOI into the ARC.
The rats were anesthetized with ketamine HCl (65 mg/kg, i.p.) and xylazine HCl (5 mg/kg, i.p.) and then placed in a stereotaxic apparatus (Kopf, Tujunga, CA). Stainless-steel guide cannulas were implanted bilaterally into the arcuate nuclei of the hypothalamus. The following coordinates were applied (Paxinos and Watson, 2007): anterior-posterior from the bregma, –2.6; lateral, ± 0.4; and ventral from the surface of the dura, –7.4. Local injections into the ARC were made through an inner cannula (ventral, –9.4) 7 days after implanting the guide cannula. Then, 5-CT (a general 5-HT1 receptor agonist) or DOI (a general 5-HT2 receptor agonist) was dissolved in 0.9% NaCl and injected into the ARC at a concentration of 10 (0.5 μl infused at a rate of 0.5 μl/min) or 6 μg/μl (0.5 μl infused at a rate of 0.5 μl/min), respectively, once a day for 5 days. The pharmacological doses of the serotonergic agonists were selected on the basis of literature data (Mamede Rosa and Prado, 1997; Willins and Meltzer, 1997; Fletcher and Korth, 1999; Fletcher et al., 2002; Currie et al., 2010; de Paula et al., 2012). Control rats were subjected to the same procedure as were the 5-HT receptor agonist–treated animals (stainless-steel guide cannulas were implanted bilaterally into the ARC), except that they received a vehicle (0.9% NaCl) instead of one of the agonists. The vehicle, 0.5 μl, or an agonist solution was administered into the ARC using a Hamilton syringe (at a flow rate of 0.5 μl/min), which was left in place for 5 minutes after injection. Two hours after the last injection the rats were sacrificed by decapitation. The position of the needle in the hypothalamus was histologically controlled in a preliminary experiment with 10 rats (using a color marker to visualize the needle track).
Collection of Pituitary, Liver, and Serum Samples.
At 2 hours after the last injection of the 5-day administration of the serotonergic agonist 5-CT or DOI, the animals were decapitated. The pituitaries and livers were isolated and stored at –80°C until they were further processed. The blood serum was separated and liver microsomes were prepared from individual animals by differential centrifugation as described previously (Daniel et al., 2000; Kot and Daniel, 2008). The obtained sera and microsomes were stored at –80°C.
Determination of Cytochrome P450 Enzyme Activities in Liver Microsomes.
The activities of cytochrome P450 enzymes in liver microsomes were measured by conducting specific metabolic reactions under linear conditions, as described previously. The activity of CYP1A was examined by measuring the rate of caffeine C-8-hydroxylation and 3-N-demethylation (Kot and Daniel, 2008). The activities of CYP2A, CYP2B, CYP2C11, and CYP3A were estimated by measuring the rate of testosterone hydroxylation in specific positions: 7α-, 16β-, 2α- and 16α-, and 2β- and 6β-hydroxylation, respectively (Haduch et al., 2006, 2008; Wójcikowski et al., 2013). The substrates and the metabolites formed were analyzed using high-performance liquid chromatography with UV detection, as described previously.
Estimation of the Cytochrome P450 Protein Levels in Liver Microsomes.
The levels of CYP2C11, CYP3A1/23, and CYP3A2 in the liver microsomes (10 μg of proteins) of control and 5-HT receptor agonist–treated rats were estimated by western immunoblot analysis, as previously described (Rysz et al., 2015, 2016a,b). Polyclonal rabbit anti-rat CYP2C11, CYP3A1/23, or CYP3A2 antibodies and a secondary antibody (a species-specific horseradish peroxidase-conjugated anti-IgG) were applied (1:2000 dilution of both primary and secondary antibodies). After incubation with a primary antibody (15 hours at 8°C), the blots were incubated with a secondary antibody (2 hours at room temperature). Rat cDNA-expressed CYP2C11 (5 µg), and CYP3A1/23 and CYP3A2 (1 µg) (Supersomes, Gentest Corp., Woburn, MA) were used as standards. The bands’ intensity on a nitrocellulose membrane was quantified with a luminescent image analyzer. The data were normalized to protein loading based on the β-actin levels (Rysz et al., 2015, 2016a,b).
Determination of the Pituitary GHRH and Serum Hormones.
Hormonal effects were measured 2 hours after the last dose of one of the serotonergic agonists. The levels of pituitary GHRH and serum hormones were measured using the following ELISA kits: GHRH kit (Elabscience); growth hormone and corticosterone kits (Demeditec); T3 and T4 kits (Cloud-Clone Corp.). Pituitaries were processed according to the manufacturer’s instructions. Briefly, pituitaries were homogenized in phosphate-buffered saline (pH = 7.0; 1:20 w/v). The homogenates were stored at –20°C for 24 hours and then centrifuged for 5 minutes at 5000g. The obtained supernatants were transferred to clean Eppendorf vials for ELISA. Absorbance was measured using a microplate reader (Rysz et al., 2015, 2016a,b).
Isolation of Liver RNA, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction Measurements.
Livers were homogenized, and total RNA was extracted using a mirVana isolation kit (Rysz et al., 2016a). The first-strand cDNA products were generated using the Transcriptor High Fidelity cDNA Synthesis Kit. The expression of genes coding for the studied cytochrome P450 enzymes (CYP2C11, CYP3A1/23, and CYP3A2) and the reference genes (HPRT1 and GAPDH) was detected by real-time polymerase chain reaction (PCR) using TaqMan Gene Expression Master Mix and species-specific TaqMan type probes and primers as described previously (Bromek et al., 2018). The gene names and identification numbers of the TaqMan primers applied in the analysis are as follows: CYP2C11 (Rn01502203_m1), CYP3A1/23 (Rn03062228_m1), CYP3A2 (Rn00756461_m1), HPRT1 (Rn01527840_m1), and GAPDH (Rn01462662_g1). Real-time PCR runs were performed using the Bio-Rad CFX96 PCR system (Bio-Rad, Hercules, CA). The PCR reactions were run in duplicate. The level of the cytochrome P450 gene’s transcripts was normalized to the HPRT1 and GAPDH genes’ expression, and the relative quantification was calculated using a comparative 2−∆∆Ct method.
Statistical Analysis of the Data.
The obtained data were statistically assessed using one-way analysis of variance followed by Duncan’s post hoc test or two-tailed Student’s t test. The results were regarded as statistically significant when P < 0.05.
Results
The expression and activity of liver cytochrome P450 were studied after the injection of pharmacological doses of the selected 5-HT1 and 5-HT2 receptor agonists into the ARC (bilaterally). The applied doses were shown to produce specific biochemical and/or behavioral effects after intracerebral administration of 5-CT (Mamede Rosa and Prado, 1997; Fletcher and Korth, 1999) or DOI (Willins and Meltzer, 1997; Currie et al., 2010; de Paula et al., 2012).
The Effect of the Local Injection of 5-HT1 and 5-HT2 Receptor Agonists into the ARC on the Activity of Cytochrome P450 in Liver Microsomes.
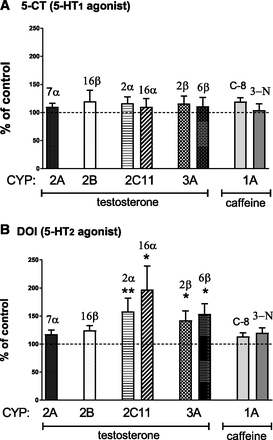
The repeated injection of 5-CT (a 5-HT1-type receptor agonist) into the ARC did not affect the activity of any cytochrome P450 enzyme studied in liver microsomes (Fig. 1A). However, in rats injected with DOI (a 5-HT2-type receptor agonist) into the ARC (3 µg/side) for 5 days, the activities of CYP2C11 and CYP3A significantly increased (Fig. 1B). The CYP2C11 activity, estimated as the rate of the 2α- and 16α-hydroxylation of testosterone, was enhanced to 164% and 207% of the control, respectively, at 2 hours after the last injection. At the same time, the activity of CYP3A, assessed as the rate of the 2β- and 6β-hydroxylation of testosterone, was elevated to 140% and 150% of the control, respectively. The activities of CYP2A (estimated as the rate of the 7α-hydroxylation of testosterone), CYP2B (evaluated as the rate of the 16β-hydroxylation of testosterone), or CYP1A (presented as the rate of caffeine C-8-hydroxylation and 3-N-demethylation) were significantly altered.
The effect of the repeated injection of the 5-HT1 receptor agonist 5-CT (A) and the 5-HT2 receptor agonist DOI (B) into the ARC (5 or 3 μg/side, respectively) on different cytochrome P450 isoenzyme activities, measured as the rates of cytochrome P450 enzyme-specific reactions in rat liver microsomes: testosterone 7α- (CYP2A), 16β- (CYP2B), 2α- and 16α- (CYP2C11), and 2β- and 6β- (CYP3A) hydroxylation and caffeine 8-hydroxylation and 3-N-demethylation (CYP1A). All values are shown as the mean ± S.E.M. (n = 8–10). Statistical significance was assessed by one-way analysis of variance followed by Duncan’s test, and is indicated as *P < 0.05; **P < 0.01, compared with the control. The control values (picomoles per milligram protein per minute) are as follows for A and B: 154.8 ± 10.4, 219.1 ± 35.0, 431.4 ± 36.9, 315.1 ± 61.4, 253.6 ± 23.9, and 810.3 ± 97.5 (testosterone 7α-, 16β-, 2α-, 16α-, 2β-, and 6β-hydroxylation, respectively), and 2.1 ± 0.1 (caffeine 8-hydroxylation), 16.0 ± 1.2 (caffeine 3-N-demethylation) for the ARC of the hypothalamus.
The Effect of the Local Injection of the 5-HT2 Receptor Agonist DOI into the ARC on the Expression of Liver Cytochrome P450.
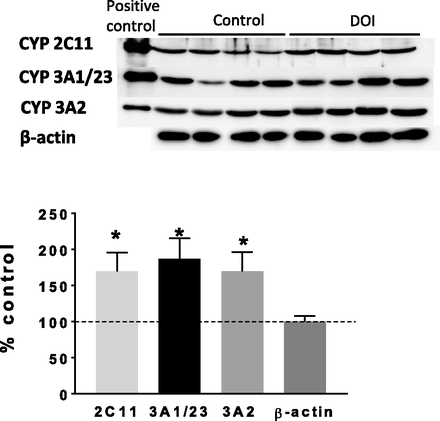
Looking for the molecular mechanisms of the 5-HT2 receptor agonist–produced increases in the activity of CYP2C11 and CYP3A, the cytochrome P450 expression was investigated in the liver after the repeated administration of DOI into the ARC. The observed increases in the activity of CYP2C11 and CYP3A correlated positively with the enzyme expression level of the respective genes observed at 2 hours after the last injection of the 5-HT2 receptor agonist DOI (Fig. 2; Table 1). DOI significantly increased the CYP2C11 protein level, CYP3A1/23, and CYP3A2 to 170%, 187%, and 170% of the control, respectively (Fig. 2). Accordingly, the CYP2C11, CYP3A1/23, and CYP3A2 mRNA levels significantly increased after the injection of DOI (Table 1). The repeated injection of the 5-HT1 agonist 5-CT into the ARC did not affect the protein levels of CYP2C11, CYP3A1/23, and CYP3A2 (Supplemental Fig. 1).
The effect of repeated injection of the 5-HT2 receptor agonist DOI into the ARC (3 μg/side) on the protein level of CYP2C11, CYP3A1/23, and CYP3A2 enzymes in rat liver microsomes. Microsomal proteins, 10 μg, were subjected to western immunoblot analysis. The presented results are representative blots from four (for the control and DOI) separate rats per treatment (A). The data are expressed as the mean ± S.E.M. (n = 6–8) (B). Statistical significance was assessed by Student’s t test and is indicated as *P < 0.05, compared with the control.
The effect of repeated injection of the 5-HT2 receptor agonist DOI into the ARC (3 μg/side) on the mRNA expression level of CYP2C11, CYP3A1/23, and CYP3A2 genes in rat liver
The results are expressed as the fold change in relation to two housekeeping genes, i.e., HPRT1 and GAPDH. All of the values are shown as the mean fold change (±S.E.M.) calculated by the comparative 2−∆∆Ct method for the control (n = 6–8) and agonist-treated (n = 6 to 7) groups. Statistical significance was assessed by Student’s t test and is indicated as P < 0.05; P < 0.01, compared with the control.
The Effect of the Local Injection of the 5-HT2 Receptor Agonist DOI into the ARC on the Level of Pituitary GHRH and Serum Hormones.
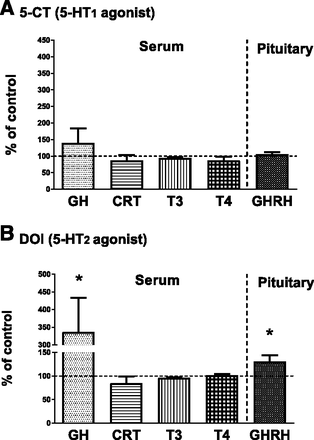
With the aim to find the central neuroendocrine mechanisms of the 5-HT2 receptor agonist DOI-evoked increases in the CYP2C11, CYP3A1/23, and CYP3A2 expression in the liver, the levels of the ARC-produced hormone GHRH and pituitary-synthesized GH were measured in the pituitary and blood serum, respectively. Moreover, the concentrations of serum corticosterone and thyroid hormones (T3 and T4), which like GH may directly regulate cytochrome P450 expression, were also analyzed (Fig. 3). The ELISA tests revealed that a repeated administration of the 5-HT2 receptor agonist DOI produced an increase in the GHRH level in the pituitary (to 137% of the control) and in the serum concentration of GH (to 330% of the control) at 2 hours after the last dose of 5-day injection of the agonist (Fig. 3B). In contrast, the injection of the 5-HT1 receptor agonist 5-CT did not increase pituitary GHRH or serum GH level. The serum concentrations of corticosterone or thyroid hormones (T3 and T4) were not affected by 5-CT or DOI.
The effect of repeated injections of the 5-HT1 receptor agonist 5-CT (A) and 5-HT2 receptor agonist DOI (B) into the ARC (5 or 3 μg/side, respectively) on the pituitary GHRH level and serum hormone concentrations. All values are presented as the mean ± S.E.M. (n = 6–9). Statistical significance was assessed by one-way analysis of variance followed by Duncan’s test and is indicated as *P < 0.05 compared with the control. The absolute control values for 5-CT and DOI were 2.9 ± 1.1, 140.8 ± 19.5, 6.7 ± 0.15, and 73.5 ± 1.8 ng/ml, and 25.6 ± 1.1 pg/mg of the tissue for serum GH, corticosterone (CRT), T3, T4, and GHRH in the pituitary, respectively.
Discussion
Our previous study carried out after local damage to the serotonergic innervation of the paraventricular or arcuate nuclei showed an important role of these hypothalamic areas in the regulation of liver cytochrome P450 via a neuroendocrine mechanism including growth hormone (Rysz et al., 2016b). Our recent study, carried out after intracerebral injection of the 5-HT1 or 5-HT2 receptor-type agonists into the PVN, allowed for identification of the serotonergic receptor subtype engaged in that neuroendocrine regulation (Bromek et al., 2018). The obtained results indicated that the serotonergic 5-HT1A receptors present in the PVN were involved in the central neuroendocrine regulation of liver cytochrome P450. The activation of the 5-HT1A receptor in the PVN produced an increase in somatostatin release and a decrease in growth hormone and corticosterone concentration in the blood, which led to a reduction in the hormone-dependent CYP2C11 and CYP3A1/23 expression and activity.
Our present study shows that in contrast to the PVN, in the ARC the 5-HT2 (but not the 5-HT1 type) receptors are involved in the neuroendocrine regulation of cytochrome P450. Moreover, the regulation of cytochrome P450 by 5-HT2 receptors of the ARC, which proceeds by the GHRH-GH hormonal pathway, is positive compared with the negative regulation of the enzyme by the 5-HT1 receptors of the PVN. The applied intracerebral dose of DOI (3 µg/side) was pharmacologically effective not only in our experiment, since it was shown also to elicit other biochemical or behavioral responses in rodents (e.g., head-twitch responses), which were inhibited by 5-HT2 receptor antagonists (Willins and Meltzer, 1997; Currie et al., 2010; de Paula et al., 2012). This corroborates our finding of the engagement of 5-HT2 receptors in the ARC in the regulation of liver cytochrome P450 expression.
Rats receiving an injection of the nonselective 5-HT2-type receptor agonist DOI into the ARC for 5 days showed increased activity of the principal cytochrome P450 enzymes in the liver of male rats, i.e., CYP2C11 and CYP3A at 2 hours after the last injection. The two reactions, namely, the 2α- and 16α-hydroxylation of testosterone, are characteristic of CYP2C11, while 2β- or 6β-hydroxylation reactions of testosterone are specific for both CYP3A enzymes (CYP3A1/23 and CYP3A2). CYP3A1/23 is expressed constitutively to a lesser degree (Gibson et al., 2002). In contrast, the repeated injection of the nonselective 5-HT1-type receptor agonist 5-CT into the ARC did not affect the activity of CYP2C11 or CYP3A.
The increased activity of the aforementioned three cytochrome P450 enzymes, produced by the repeated injection of the 5-HT2 receptor agonist DOI into the ARC, correlated positively with the enzyme expression level (mRNA and protein) in the liver. DOI significantly enhanced the mRNA levels of CYP2C11, CYP3A1/23, and CYP3A2 genes and their respective protein products in the liver. This indicates that the activation of the 5-HT2-type receptor in the ARC stimulates the synthesis of the liver CYP2C11, CYP3A1/23, and CYP3A2 protein at the transcriptional level, and this effect is opposite to that observed previously after the bilateral damage to the serotonergic innervation of the ARC (Rysz et al., 2016b).
Liver cytochrome P450 is regulated hormonally by pituitary-produced growth hormone, adrenal corticosterone, and thyroid hormones (T3 and T4). In our experiment, 5-HT2 receptor agonist–evoked increases in cytochrome P450 expression and activity were accompanied by elevation of the pituitary GHRH and serum GH with no change in serum concentrations of corticosterone or thyroid hormones. Growth hormone is the main physiologic factor stimulating the expression of the CYP2C11 gene in male rats due to its pulsatile secretion by the anterior pituitary gland (Waxman and O’Connor, 2006; Waxman and Holloway, 2009). A good positive correlation between the serum GH concentration and the protein level and activity of CYP2C11 in the liver was found in male rats (Kot et al., 2015). Moreover, apart from corticosterone, the main CYP3A regulator (Gibson et al., 2002; Dvorak and Pavek, 2010), GH can also positively regulate the CYP3A subfamily enzymes (Waxman et al., 1995; Waxman and Holloway, 2009). A positive correlation between the GH concentration and the CYP3A activity was observed in the liver of male rats (Kot et al., 2015). Thus, the observed increase in the expression of liver CYP2C11, CYP3A1/23, and CYP3A2 may be connected to the enhanced GH concentration in blood serum. An alteration of the temporal pattern of serum GH (a shift from pulsatile to continuous secretion) would possibly produce a decrease, rather than an increase, in the expression and activity of these enzymes in our experiment (Waxman and O’Connor, 2006).
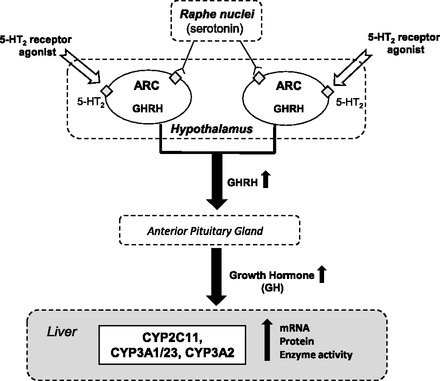
In conclusion, the injection of the selected 5-HT1- and 5-HT2-type receptor agonists at pharmacologically active doses into the arcuate nuclei of the hypothalamus, followed by the estimation of pituitary GHRH, serum hormone levels, and liver cytochrome P450 expression, showed a new brain serotonergic mechanism engaged in the neuroendocrine regulation of liver cytochrome P450 (Fig. 4). The collected data indicate that the serotonergic receptors of 5-HT2 type, but not of 5-HT1 type, present in the ARC are involved in the central neuroendocrine regulation of cytochrome P450 in the liver. The activation of the 5-HT2 receptor in the ARC produces an increase in GHRH release and pituitary growth hormone secretion, which possibly leads to an elevation in the hormone-dependent CYP2C11, CYP3A1/23, and CYP3A2 genes’ expression and activity in the liver. Since 5-HT2 receptors are engaged in the mechanisms of pharmacological action of central nervous system drugs (e.g., atypical neuroleptics, antidepressants, or antiobesity drugs), treatment with these drugs may affect the physiologic regulation of hormone-dependent cytochrome P450 enzymes that take part in the metabolism of endogenous substrates and other coadministered drugs (Meltzer and Massey, 2011; Quesseveur et al., 2012; Rosenzweig-Lipson et al., 2012; Jensen et al., 2010; Nigro et al., 2013). However, further studies are necessary to identify which of the 5-HT2 receptor subtypes (5-HT2A, 5-HT2B, or 5-HT2C) present in the ARC is responsible for the observed central neuroendocrine regulation of cytochrome P450 expression in the liver.
Neuroendocrine regulation of liver cytochrome P450 by serotonergic receptor of the 5-HT2 type located in the ARC (the scheme depicts the results presented in this work). The activation of the 5-HT2-type receptor in the ARC of the hypothalamus stimulates GHRH release and thus pituitary GH secretion, which may lead to increased expression and activity of the GH-dependent CYP2C11, CYP3A1/23, and CYP3A2 enzymes in the liver.
Authorship Contributions
Participated in research design: Daniel.
Conducted experiments: Bromek, Rysz, Haduch.
Performed data analysis: Bromek, Rysz, Haduch, Daniel.
Wrote or contributed to the writing of the manuscript: Daniel, Bromek, Rysz.
Footnotes
- Received August 1, 2018.
- Accepted November 30, 2018.
This study was financially supported by the National Science Centre, Kraków, Poland [OPUS 6 Grant 4 2013/11/B/NZ7/04897] and by statutory funds from the Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- ARC
- arcuate nucleus
- 5-CT
- 5-carboxyamidotryptamine
- DOI
- 2,5-dimethoxy-4-iodoamphetamine
- ELISA
- enzyme-linked immunosorbent assay
- GH
- growth hormone
- GHRH
- growth hormone–releasing hormone
- 5-HT
- serotonin
- PCR
- polymerase chain reaction
- PVN
- paraventricular nucleus
- T3
- triiodothyronine
- T4
- thyroxine
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics