Abstract
Prasugrel, a thienopyridine anti-platelet agent, is pharmacologically activated by hydrolysis and hydroxylation. It is efficiently hydrolyzed in the intestine after oral administration, and the enzyme responsible for the hydrolysis in humans was demonstrated to be carboxylesterase (CES)2. Prasugrel hydrolase activity is detected in dog intestines, where CES enzymes are absent; therefore, this prompted us to investigate the involvement of an enzyme(s) other than CES. Human arylacetamide deacetylase (AADAC) is highly expressed in the small intestine, catalyzing the hydrolysis of several clinical drugs containing small acyl moieties. In the present study, we investigated whether AADAC catalyzes prasugrel hydrolysis. Recombinant human AADAC was shown to catalyze prasugrel hydrolysis with a CLint value of 50.0 ± 1.2 ml/min/mg protein with a similar Km value to human intestinal and liver microsomes, whereas the CLint values of human CES1 and CES2 were 4.6 ± 0.1 and 6.6 ± 0.3 ml/min/mg protein, respectively. Inhibition studies using various chemical inhibitors and the relative activity factor approach suggested that the contribution of AADAC to prasugrel hydrolysis in human intestine is comparable to that of CES2. In dog intestine, the expression of AADAC, but not CES1 and CES2, was confirmed by measuring the marker hydrolase activities of each human esterase. The similar Km values and inhibition profiles between recombinant dog AADAC and small intestinal microsomes suggest that AADAC is a major enzyme responsible for prasugrel hydrolysis in dog intestine. Collectively, we found that AADAC largely contributes to prasugrel hydrolysis in both human and dog intestine.
Introduction
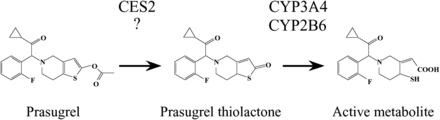
Prasugrel, a thienopyridine anti-platelet agent, is used for patients with ischemic cardiac events who are undergoing percutaneous coronary intervention. It is a prodrug that is activated by hydrolysis and hydroxylation in the body. Previous reports have demonstrated that after oral administration to humans, prasugrel is efficiently hydrolyzed to prasugrel thiolactone by carboxylesterase (CES)2 in the intestine, followed by oxidization to an active metabolite by cytochrome P450 (CYP)3A4 and CYP2B6 in the intestine and liver (Rehmel et al., 2006; Farid et al., 2007; Williams et al., 2008) (Fig. 1). A previous report has demonstrated the benefit of prasugrel, in that its platelet inhibition effects showed faster onset and less interindividual variability than clopidogrel (Payne et al., 2007) because ∼85% of the absorbed clopidogrel is rapidly hydrolyzed to an inactive metabolite by CES1 (Hagihara et al., 2009).
Metabolic pathways of prasugrel and responsible enzymes in humans. A previous study demonstrated that prasugrel is hydrolyzed by CES2. Because prasugrel is hydrolyzed in dog intestine where CES2 is not expressed, the involvement of an enzyme(s) other than CES2 is presumed.
CES enzymes are involved in the hydrolysis of various types of drugs and xenobiotics (Fukami and Yokoi, 2012). Although CES enzymes are divided into five families in mammals, the CES families involved in drug metabolism are mainly CES1 and CES2 (Imai et al., 2006; Holmes et al., 2010); CES1 is mainly expressed in the liver and CES2 is highly expressed in the intestine and liver (Imai, 2006). In the case of human CES1 and CES2, substrate specificities are generally defined depending on the size of the acyl and alcohol/amine moieties in the compounds (Fukami and Yokoi, 2012); CES1 prefers substrates containing large acyl and small alcohol/amine moieties, whereas CES2 prefers substrates containing large alcohol/amine and small acyl moieties. The chemical structure of prasugrel fits the characteristics of CES2 substrates.
In the processes of drug development, prediction of drug disposition in humans using experimental animals is usually adopted to estimate pharmacological effects and toxicity of drug candidates. However, species differences in drug-metabolizing enzymes sometimes make the extrapolation to humans difficult. In dogs, both CES1 and CES2 are expressed in the liver but not in the intestine (Taketani et al., 2007). Although substrates of CES enzymes are predicted to be unlikely hydrolyzed in the dog intestine, a previous study reported that the dog intestinal S9 fraction showed prasugrel hydrolase activity comparable to the human intestinal S9 fraction (Hagihara et al., 2011). This result made us hypothesize that enzyme(s) other than CES2 are involved in prasugrel hydrolysis.
Arylacetamide deacetylase (AADAC), which is expressed in the gastrointestinal tract and liver, is involved in the metabolism of various drugs. To date, it has been shown that human AADAC catalyzes the hydrolysis of flutamide, phenacetin, rifamycins, and indiplon (Watanabe et al., 2009, 2010; Nakajima et al., 2011; Shimizu et al., 2014a). Recent studies have uncovered that human AADAC prefers compounds containing a quite small acyl moiety (e.g., Fukami et al., 2015). Based on the chemical structure of prasugrel (Fig. 1), we surmised that AADAC catalyzes prasugrel hydrolysis.
Identification of enzyme(s) involved in the metabolism of a drug is required to estimate the interindividual variability of drug efficacy and drug-drug interactions. In the present study, we investigated whether human AADAC can catalyze prasugrel hydrolysis and whether the efficient hydrolysis of prasugrel in the dog intestine, where CES enzymes are not expressed, might be explained by AADAC. We found that both human and dog AADAC can catalyze prasugrel hydrolysis, and that AADAC is an enzyme responsible for high efficient hydrolysis of prasugrel in the dog intestine.
Materials and Methods
Chemicals and Reagents.
Prasugrel hydrochloride was purchased from Toronto Research Chemicals (Toronto). Phenylmethylsulfonyl fluoride (PMSF), diisopropyl fluorophosphate (DFP), and eserine sulfate were purchased from Wako Pure Chemical Industries (Osaka, Japan). Telmisartan was obtained from LKT Laboratories (St. Paul, MN). Human intestinal (pooled human intestinal microsomes (HIMs), n = 7) and liver microsomes [pooled human liver microsomes (HLMs), n = 50] were purchased from Corning (Corning, NY). Dog intestinal [pooled dog intestinal microsomes (DIMs), n = 3, male] and liver microsomes [pooled dog liver microsomes (DLMs), n = 8, male] were obtained from Xenotech, LLC (Lenexa, KS). All primers were commercially synthesized at Greiner Japan (Tokyo) or Sigma-Aldrich (St. Louis, MO). All other chemicals were of the highest commercially available grade.
Recombinant Esterases Expressed in Sf21 Cells.
Sf21 cell homogenates expressing human AADAC, CES1, and CES2 were previously prepared (Fukami et al., 2010; Watanabe et al., 2010). An expression system of dog AADAC was constructed using a Bac-to-Bac Baculovirus Expression System (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Dog AADAC cDNA was obtained by reverse transcription-polymerase chain reaction using a dog liver RNA sample (UNITECH, Chiba, Japan) with AADAC EcoRI (5′-GGGAATTCGTGTTGCCAAGTCAAGAGAG-3′) and AADAC XhoI (5′-CCCCTCGAGCCCCTTCCTCAGATTTTTAC-3′) primers. The nucleotide sequences (accession no. XM_534309) were confirmed by DNA sequence analysis (FASMAC, Kanagawa, Japan). The cDNA was transferred into the pFastBac1 vector and digested with EcoRI and XhoI. The pFastBac1 vector containing AADAC cDNA was transformed into DH10Bac-competent cells, followed by transposition of the inserts into bacmid DNA. Nonrecombinant bacmid DNA (mock) was also prepared by the same procedures. Other steps including transfection of bacmid DNA into Spodoptera frugiperda Sf21 cells (Invitrogen) and preparation of cell homogenates were performed according to the method described previously (Iwamura et al., 2012). Protein concentrations were determined according to the method of Bradford (1976) using γ-globulin as the standard.
Prasugrel Hydrolase Activity.
Prasugrel hydrolase activities were determined as follows: a typical incubation mixture (final volume of 0.2 ml) contained 100 mM potassium phosphate buffer (pH 7.4) and enzyme sources (HLMs, 0.01 mg/ml; HIMs, 0.01 mg/ml; DLMs, 0.005 mg/ml; DIMs, 0.03 mg/ml; Sf21 cell homogenates expressing human AADAC, 0.01 mg/ml; CES1, 0.01 mg/ml; CES2, 0.01 mg/ml; and dog AADAC, 0.005 mg/ml). After 2-minute preincubation at 37°C, the reactions were initiated by the addition of prasugrel (the final concentration was 5 μM). Prasugrel hydrochloride was dissolved in dimethylsulfoxide (DMSO). The final concentration of DMSO in the incubation mixture was 2%. After 1-minute incubation, the reactions were terminated by addition of 100 μl of ice-cold acetonitrile. In a preliminary study, we confirmed that the formation rates of prasugrel thiolactone from prasugrel were linear with respect to protein concentration (<0.02 mg/ml HIMs, 0.015 mg/ml HLMs, 0.04 mg/ml DIMs, and 0.006 mg/ml DLMs) and incubation time <1.5 minutes. A 50 μl portion of the supernatant obtained by centrifugation at 12,000g for 5 minutes was subjected to high-performance liquid chromatography (HPLC). The HPLC analysis was performed using an L-2130 pump (Hitachi, Tokyo), an L-2200 autosampler (Hitachi), an L-2400 UV detector (Hitachi), and a D-2500 Chromato-Integrator (Hitachi) equipped with an Inertsil-ODS3 column (5 μm particle size, 4.6 mm i.d. × 250 mm; GL Science, Tokyo). The eluent was monitored at 254 nm using a UV detector equipped with noise-base clean Uni-3 (Union, Gunma, Japan), which can reduce the noise by integrating the output and can increase the signal 3-fold by differentiating the output and 5-fold by further amplification with an internal amplifier, resulting in a maximum 15-fold amplification of the signal. The mobile phase was 70% acetonitrile containing 5 mM potassium phosphate buffer (pH 7.4). The flow rate was 0.6 ml/min. The column temperature was 35°C. Because prasugrel is nonenzymatically hydrolyzed to some extent, the content of prasugrel thiolactone in the mixture incubated without the enzyme sources was subtracted from that with the enzyme sources. The quantification of prasugrel thiolactone was performed as described subsequently.
Identification and Quantification of Prasugrel Thiolactone.
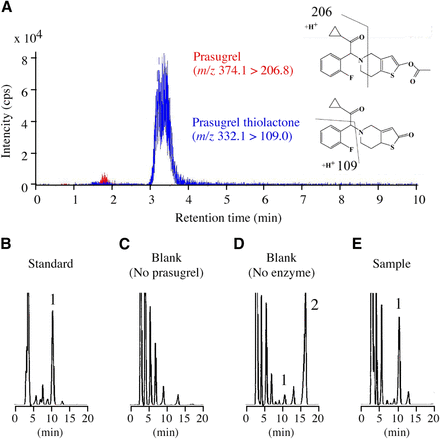
Because the authentic standard of prasugrel thiolactone is not commercially available, liquid chromatography–tandem mass spectrometry analysis was performed to determine whether the concerned peak represents prasugrel thiolactone. The liquid chromatography equipment comprised an HP1100 system with a binary pump, an automatic sampler, and a column oven (Agilent Technologies, Santa Clara, CA) equipped with ZORBAX (3.5 μm particle size, 2.1 mm i.d. × 50 mm; Agilent Technologies). The column temperature was 25°C. The mobile phase was 55% methanol with 0.1% formic acid. The flow rate was 0.2 ml/min. The liquid chromatography equipment was connected to a PE Sciex API2000 tandem mass spectrometer (ABSciex, Framingham, MA), which was operated in the positive electrospray ionization mode. In the multiple-reaction monitoring mode, the ion transitions of m/z 332.1 > 109.0 for prasugrel thiolactone and m/z 374.1 > 206.8 for prasugrel were monitored. Turbo gas was maintained at 550°C. Nitrogen was used as the nebulizing, turbo, and curtain gases at 60, 85, and 40 psi, respectively. The collision energy was 25 V. The analytical data were processed using the Analyst software (version 1.5; Applied Biosystems, Foster City, CA). After injection of samples incubated with prasugrel and HLMs to liquid chromatography–tandem mass spectrometry, the fraction of prasugrel thiolactone confirmed with multiple-reaction monitoring (Fig. 2A) was collected, concentrated, and subjected to HPLC as described previously (Fig. 2B). A HPLC peak detected with a sample incubated with HLM and 10 μM prasugrel for 15 minutes showed the same retention time to that collected from liquid chromatography–tandem mass spectrometry (Fig. 2, B–E), indicating that prasugrel was efficiently hydrolyzed to prasugrel thiolactone.
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) and HPLC chromatograms of prasugrel and prasugrel thiolactone. (A) In the multiple-reaction monitoring (MRM) mode, the ion transitions of m/z 374.1 > 206.8 for prasugrel and m/z 332.1 > 109.0 for prasugrel thiolactone were monitored. Prasugrel (100 μM) was incubated with HLMs for 60 minutes, and the incubation mixtures were subjected to LC-MS/MS. (B) The fraction of prasugrel thiolactone confirmed with MRM was injected to HPLC. The eluent was monitored at 254 nm. (C) HLMs (0.1 mg/ml), (D) prasugrel (10 μM), and (E) HLMs and prasugrel were incubated at 37°C for 15 minutes and then analyzed with HPLC. Peak 1, prasugrel thiolactone; Peak 2, prasugrel.
The formed prasugrel thiolactone was quantified by HPLC analysis based on the decreased amount of prasugrel, according to a method described in a previous study for indiplon hydrolysis (Shimizu et al., 2014a). Briefly, prasugrel at concentrations of 1 and 10 μM was incubated with HLMs (0.1 mg/ml). After 15-minute incubation, prasugrel was completely converted to prasugrel thiolactone (Fig. 2, D and E). The amounts of prasugrel were assigned for the formed prasugrel thiolactone. After determining the peak height per known content of prasugrel thiolactone, the value was used to calculate hydrolase activity of prasugrel.
Kinetic Analyses of Prasugrel Hydrolysis in Tissue Microsomes and Recombinant Esterases.
Kinetic analyses of prasugrel hydrolysis were performed at ranges of 1–80 μM of prasugrel concentrations. The parameters were estimated from the fitted curves using a computer program (KaleidaGraph, Synergy Software, Reading, PA) designed for nonlinear regression analyses.
Inhibition Studies of Prasugrel Hydrolase Activity.
To confirm the involvement of AADAC in prasugrel hydrolysis in humans and dogs, inhibition studies with HIM, HLM, DIM, and recombinant dog AADAC were performed using representative esterase inhibitors including DFP, PMSF, eserine, and telmisartan. Final concentrations of the inhibitors were 100 μM (DFP, PMSF) or 10 μM (eserine, telmisartan). DFP, a type of organophosphate, is known to potently inhibit CES and AADAC at 100 μM (Tabata et al., 2004; Watanabe et al., 2009). PMSF is a general inhibitor of serine esterases and potently inhibits CES enzymes at 100 μM (Fujiyama et al., 2010) but does not inhibit AADAC (Watanabe et al., 2009; Shimizu et al., 2014b). Eserine potently inhibits CES2 and AADAC at 10 μM (Kobayashi et al., 2012a). Telmisartan specifically inhibits CES2 at 10 μM (Shimizu et al., 2014b). DFP, PMSF, and telmisartan were dissolved in DMSO such that the final concentration of DMSO in the incubation mixture was 2%. Eserine was dissolved in distilled water. The experimental procedures and conditions were the same as described previously. It was confirmed that 2% DMSO did not inhibit prasugrel hydrolase activity, and the control activity was determined in the presence of 2% DMSO.
Contributions of AADAC, CES1, and CES2 to Prasugrel Hydrolase Activity in HIMs and HLMs.
The percentage contributions of AADAC, CES1, and CES2 to the prasugrel hydrolase activity in HIMs and HLMs were estimated by applying the relative activity factor (RAF) as the ratio of activity values, as described previously (Watanabe et al., 2010). In this study, the RAF values for AADAC (RAFAADAC,HIM or RAFAADAC,HLM) were determined as the ratios of the indiplon hydrolase activities in HIMs and HLMs to the value by recombinant human AADAC. The RAF values for CES1 (RAFCES1,HIM or RAFCES1,HLM) were determined as the ratios of the fenofibrate hydrolase activities in HIMs and HLMs to the value by recombinant human CES1. The RAF values for CES2 (RAFCES2,HIM or RAFCES2,HLM) were determined as the ratios of the procaine hydrolase activities in HIMs and HLMs to the value by recombinant human CES2. Using the RAF values, the predicted prasugrel hydrolase activities by AADAC (VAADAC,HIM or VAADAC,HLM), CES1 (VCES1,HIM or VCES1,HLM), and CES2 (VCES2,HIM or VCES2,HLM) in HIMs or HLMs were calculated as follows: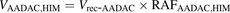 (1)
(1)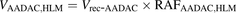 (2)
(2)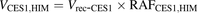 (3)
(3)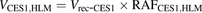 (4)
(4)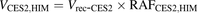 (5)
(5)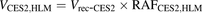 (6)where Vrec-AADAC, Vrec-CES1, and Vrec-CES2 are the prasugrel hydrolase activities by recombinant AADAC, CES1, and CES2, respectively. The contributions of AADAC, CES1, and CES2 to the prasugrel hydrolase activities in HIMs or HLMs were calculated using the following equations:
(6)where Vrec-AADAC, Vrec-CES1, and Vrec-CES2 are the prasugrel hydrolase activities by recombinant AADAC, CES1, and CES2, respectively. The contributions of AADAC, CES1, and CES2 to the prasugrel hydrolase activities in HIMs or HLMs were calculated using the following equations: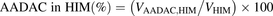 (7)
(7)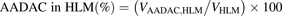 (8)
(8)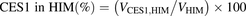 (9)
(9)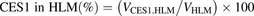 (10)
(10)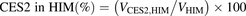 (11)
(11)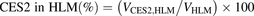 (12)where the VHIM and VHLM values are the observed prasugrel hydrolase activities in HIMs and HLMs, respectively.
(12)where the VHIM and VHLM values are the observed prasugrel hydrolase activities in HIMs and HLMs, respectively.
Indiplon, Phenacetin, Fenofibrate, and Procaine Hydrolase Activity.
Indiplon, phenacetin, fenofibrate, and procaine hydrolase activities were measured according to previous studies (Watanabe et al., 2010; Shimizu et al., 2014a; Fukami et al., 2015). Their substrate concentrations were 1 mM, 5 mM, 25 μM, and 5 mM, respectively.
Results
Kinetic Analyses of Prasugrel Hydrolase Activity by Recombinant Human Esterases, HIMs, and HLMs.
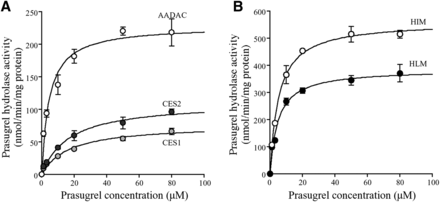
Prasugrel hydrolysis was reported to be mainly catalyzed by CES2 in human intestine (Williams et al., 2008). To investigate whether CES2 is the sole enzyme involved in this hydrolysis, we performed kinetic analyses of prasugrel hydrolase activity using recombinant human AADAC, CES1, and CES2. The activity was detected by all recombinant human esterases, and data for activities followed the Michaelis-Menten equations (Fig. 3A). The Km, Vmax, and CLint values for prasugrel hydrolysis by recombinant human AADAC were 4.6 ± 0.2 μM, 228.4 ± 13.6 nmol/min/mg protein, and 50.0 ± 1.2 ml/min/mg protein, respectively. In human CES1 and CES2, the Km values were higher and the Vmax values were lower than those in human AADAC (Table 1). Thus, human AADAC showed the highest catalytic efficiency among the three recombinant esterases, although we cannot directly compare the Vmax values because expression levels may be different in expression systems. This is the first study to report the kinetics of prasugrel hydrolysis in HLMs and HIMs. The Km, Vmax, and CLint values in HIMs were 5.3 ± 0.2 μM, 560.6 ± 20.7 nmol/min/mg protein, and 106.4 ± 1.4 ml/min/mg protein, respectively, and those in HLMs were 4.6 ± 0.5 μM, 382.0 ± 27.9 nmol/min/mg protein, and 83.9 ± 3.1 ml/min/mg protein, respectively (Fig. 3B; Table 1). Because the Km values in HIMs and HLMs were similar to that of recombinant human AADAC, it was suggested that AADAC would be one of the responsible enzymes for prasugrel hydrolysis in human intestine and liver.
Kinetic analyses of prasugrel hydrolase activity by recombinant human esterases and human tissue microsomes. (A) Recombinant human AADAC, CES1, or CES2 (0.01 mg/ml), and (B) HIMs or HLMs (0.01 mg/ml) were incubated with prasugrel for 1 minute. Prasugrel hydrolase activity was measured by HPLC. Each point represents the mean ± S.D. of triplicate determinations.
Kinetic parameters of prasugrel hydrolysis by recombinant esterases and human tissue microsomes
Effects of Chemical Inhibitors on Prasugrel Hydrolase Activities in HIMs and HLMs.
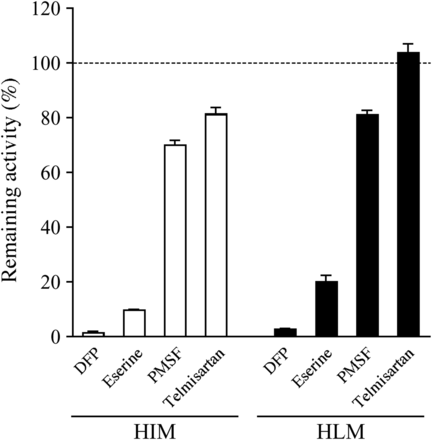
To investigate the contribution of each esterase to prasugrel hydrolysis in human intestine and liver, the effects of various chemical inhibitors on prasugrel hydrolase activities in HIMs and HLMs were determined (Fig. 4). The prasugrel hydrolase activities in HIMs and HLMs were efficiently inhibited by DFP and eserine but were slightly inhibited by PMSF. Although telmisartan slightly inhibited the activity in HIMs, it did not inhibit the activity in HLMs. These results suggest that AADAC is involved in prasugrel hydrolysis in human intestine and liver.
Inhibitory effects of chemical inhibitors on prasugrel hydrolase activity. HIMs or HLMs (0.01 mg/ml) were incubated with 5 μM prasugrel for 1 minute. The control activity values by HIMs and HLMs were 267.5 ± 12.5 and 248.0 ± 13.8 nmol/min/mg protein, respectively. Each column represents the mean ± S.D. of triplicate determinations.
Contribution of AADAC, CES1, and CES2 to Prasugrel Hydrolase Activity in HIMs and HLMs.
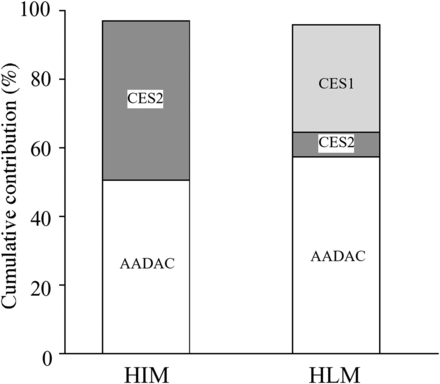
To estimate the contribution of AADAC, CES1, and CES2 to prasugrel hydrolase activity in HIMs and HLMs, RAF values for each esterase in HIMs and HLMs were calculated (Table 2). We previously demonstrated that indiplon, fenofibrate, and procaine were specifically hydrolyzed by AADAC, CES1, and CES2, respectively (Shimizu et al., 2014a; Fukami et al., 2015). Therefore, we selected these compounds as the specific substrates of each esterase. The values of indiplon hydrolase activity at 1 mM in HIMs and HLMs were 221.0 and 338.2 pmol/min/mg protein, respectively. Using the activity of recombinant AADAC (265.0 pmol/min/mg protein), the RAFAADAC,HIM and RAFAADAC,HLM values were calculated as 0.83 and 1.27, respectively. The value of fenofibrate hydrolase activity at 25 μM in HLMs was 421.4 nmol/min/mg protein, whereas the activity was not detected in HIMs. Using the activity of recombinant CES1 (84.0 nmol/min/mg protein), the RAFCES1,HLM value was calculated as 5.01. The values of procaine hydrolase activity at 5 mM in HIMs and HLMs were 11.1 and 2.3 nmol/min/mg protein, respectively. Using the activity of recombinant CES2 (4.3 nmol/min/mg protein), the RAFCES2,HIM and RAFCES2,HLM values were calculated as 2.60 and 0.54, respectively. The contribution ratio of each esterase in HIMs and HLMs was estimated according to the equations described in Materials and Methods, resulting in AADAC, CES1, and CES2 values in HIMs of 50.6%, 0%, and 46.4%, respectively, and those in HLMs of 57.3%, 31.6%, and 7.1%, respectively. The cumulative contribution of the three enzymes is illustrated in Fig. 5. The total contributions of AADAC, CES1, and CES2 in HIMs and HLMs were 97.0% and 96.0%, respectively. It was shown that AADAC is one of the enzymes highly contributing to prasugrel hydrolysis.
RAF values calculated from the marker activity and the contributions of AADAC, CES1, and CES2 to prasugrel hydrolysis in HIMs and HLMs
Contribution of each esterase to prasugrel hydrolysis in HIMs and HLMs. Extrapolation was determined by the RAF using HIMs, HLMs, and recombinant human enzymes. The prasugrel concentration used in this study was 5 μM.
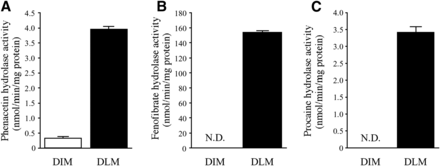
Hydrolase Activity of Phenacetin, Fenofibrate, and Procaine in Dog Tissue.
We surmised that the hydrolysis of prasugrel in dog intestine might be catalyzed by AADAC. Because it was unknown whether AADAC is expressed in dog intestine, we sought to measure the hydrolase activity of indiplon in DLMs and DIMs as a marker activity of AADAC. However, recombinant dog AADAC hardly showed indiplon hydrolysis activity at 1 mM substrate concentration (data not shown). As a second marker substrate of AADAC, we selected phenacetin because recombinant dog AADAC showed high activity toward phenacetin hydrolysis (5.4 ± 0.0 nmol/min/mg protein at 5 mM substrate concentration). The hydrolase activity at 5 mM phenacetin was detected in DIMs (0.3 ± 0.1 nmol/min/mg protein), although the value was much lower than that in DLMs (4.0 ± 0.0 nmol/min/mg protein) (Fig. 6A). On the other hand, the hydrolase activities of fenofibrate and procaine, which were human CES1 and CES2 marker substrates, respectively, were not detected in DIMs (Fig. 6, B and C), although these activities were highly detected in DLMs (153.9 ± 1.5 and 3.4 ± 0.2 nmol/min/mg protein, respectively). We confirmed that recombinant dog AADAC did not show fenofibrate and procaine hydrolase activity (data not shown). These results indicate that AADAC is functionally expressed in dog intestine.
Hydrolase activities of phenacetin, fenofibrate, and procaine in DIMs and DLMs. DIMs or DLMs [(A) 0.4 mg/ml, (B) 0.025 mg/ml, and (C) 0.5 mg/ml] were incubated with (A) 5 mM phenacetin specific for AADAC, (B) 5 μM fenofibrate specific for CES1, and (C) 1 mM procaine specific for CES2. Each column represents the mean ± S.D. of triplicate determinations; N.D., not detected.
Kinetic Analyses of Prasugrel Hydrolase Activity by Recombinant Dog AADAC and DIMs.
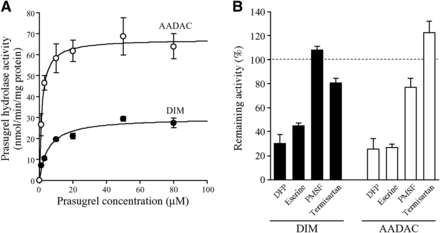
To investigate whether dog AADAC can catalyze prasugrel hydrolysis, kinetic analysis of prasugrel hydrolase activity by DIMs and recombinant dog AADAC was performed (Fig. 7A; Table 3). Data for the activity in DIMs followed the Michaelis-Menten equations, and the Km, Vmax, and CLint values were 5.3 ± 0.6 μM, 29.8 ± 2.0 nmol/min/mg protein, and 5.6 ± 0.3 ml/min/mg protein, respectively (Table 3). The activity was also detected by recombinant dog AADAC, and data for the activity followed the Michaelis-Menten equations. The Km, Vmax, and CLint values were 1.5 ± 0.1 μM, 67.4 ± 6.5 nmol/min/mg protein, and 45.9 ± 3.1 ml/min/mg protein, respectively (Table 3). Thus, recombinant dog AADAC showed a similar Km value to DIMs, suggesting that AADAC is the major enzyme involved in prasugrel hydrolysis in dog intestine.
(A) Kinetic analyses of prasugrel hydrolase activity by DIMs and recombinant dog AADAC. DIMs (0.03 mg/ml) or recombinant dog AADAC (0.005 mg/ml) were incubated with prasugrel for 1 minute. Prasugrel hydrolase activity was measured by HPLC. Each point represents the mean ± S.D. of triplicate determinations. (B) Inhibitory profile of chemical inhibitors against prasugrel hydrolase activity. DIMs (0.03 mg/ml) or recombinant dog AADAC (0.005 mg/ml) were incubated with 5 μM prasugrel for 1 minute. The control activity values by DIMs and recombinant dog AADAC were 9.55 ± 0.14 and 4.23 ± 0.13 nmol/min/mg protein, respectively. Each column represents the mean ± S.D. of triplicate determinations.
Kinetic parameters of prasugrel hydrolysis by DIMs and dog AADAC
Effects of Chemical Inhibitors on Prasugrel Hydrolase Activity by DIMs and Recombinant Dog AADAC.
To support the major contribution of AADAC to prasugrel hydrolysis in dog intestine, the inhibition profiles of some inhibitors were compared between DIMs and recombinant dog AADAC (Fig. 7B). The hydrolase activity in DIMs was potently inhibited by DFP and eserine but was not inhibited by PMSF and telmisartan. The prasugrel hydrolase activity by recombinant dog AADAC was also potently inhibited by DFP and eserine but was not inhibited by PMSF and telmisartan. This inhibitory characteristic was similar to that in human tissues (Fig. 4). Collectively, these results support that AADAC is major contributor to prasugrel hydrolysis in dog intestine.
Discussion
Prasugrel is efficiently hydrolyzed to prasugrel thiolactone in the intestine after oral administration, followed by oxidation to an active metabolite by CYP3A4 and CYP2B6 in the intestine and liver (Rehmel et al., 2006; Farid et al., 2007; Williams et al., 2008). It has been believed that prasugrel hydrolysis was catalyzed by CES2 in humans (Williams et al., 2008). High prasugrel hydrolase activity was observed in dog intestine (Hagihara et al., 2011), even though CES2 protein is not expressed in dog intestine (Taketani et al., 2007). This fact implies a possibility that enzyme(s) other than CES enzymes would catalyze prasugrel hydrolysis. Because we have recently found that AADAC prefers compounds containing small acyl moieties, we expected that AADAC would be involved in the hydrolysis of prasugrel, which has a small acyl moiety.
First, we performed kinetic analyses of prasugrel hydrolase activity by recombinant human AADAC, CES1, and CES2 (Fig. 3A), showing that the highest catalytic potency was detected in human AADAC (Table 1). The Km value obtained by recombinant human AADAC was similar to those in HIMs and HLMs (Fig. 3B; Table 1). HIMs and HLMs were selected for this study because the intestine is a main organ for prasugrel hydrolysis, and the liver is an organ that metabolizes a majority of drugs. The results suggested that AADAC is involved in prasugrel hydrolysis in human intestine and liver. The high CLint value in HIMs suggests that a large portion of prasugrel was hydrolyzed in the intestine after oral administration, as previously reported (Hagihara et al., 2011). The Km value by recombinant CES1 in this study (16.7 ± 1.9 μM) was similar to that reported in a previous study using purified CES1 (9.3 ± 0.8 μM) (Williams et al., 2008). Data for the prasugrel hydrolysis by recombinant human CES2 also fit the Michaelis-Menten equation, with a Km value of 17.1 ± 1.8 μM in our study (Fig. 3A; Table 1). However, Williams et al. (2008) reported that data for human CES2 fit a Hill equation at prasugrel concentrations <40.5 μM and an inhibition plot at concentrations >40.5 μM. This inconsistency may be due to the difference in the enzyme sources (homogenates of Sf21 cells expressing human CES2 versus purified human CES2).
We performed inhibition studies using several inhibitors to estimate the contribution of AADAC to prasugrel hydrolysis in HIMs and HLMs (Fig. 4). The activities in both HIMs and HLMs were strongly inhibited by DFP and eserine, which are known to inhibit AADAC activity. In addition, the inhibition profiles were similar between HIMs and HLMs. These results suggest that AADAC highly contributes to prasugrel hydrolysis in HIMs and HLMs. Telmisartan, a specific inhibitor of human CES2, showed the inhibitory effect on only HIMs, although the effect was slight. The results suggest that the contribution of CES2 to prasugrel hydrolysis in HIMs would be higher than that in HLMs.
To further quantitatively elucidate the contribution of each esterase in HIMs, we used the RAF approach. The concept of RAF was proposed by Crespi (1995) to extrapolate the data of recombinant CYP enzymes to the activity in human liver. To calculate the RAF, the V value was used. The RAF using the V value requires an appropriate substrate concentration to obtain a reasonable prediction (Crespi and Miller, 1999). In this study, we selected concentrations approximately 5-fold higher than the Km value for marker substrates to calculate the RAF values. As a result, it was estimated that AADAC showed similar or higher contribution ratios in the intestine compared with CES2 (Fig. 5; Table 2). Although hydrolysis in human liver hardly appears to contribute to pharmacological activation of prasugrel in the body owing to the high clearance in human intestine, the contribution ratio of each esterase in HLMs was also estimated by the RAF approach. CES1 is predominantly expressed in human liver (Imai, 2006) and highly contributes to the hydrolysis of many compounds. Nevertheless, AADAC showed the highest contribution to prasugrel hydrolysis in the liver. Considering the results of the inhibition study (Fig. 4) and the RAF approach (Fig. 5) together, AADAC plays a strong role in prasugrel hydrolysis in humans.
Next, we investigated whether prasugrel hydrolysis in dog intestine might be catalyzed by dog AADAC. Because it had been unknown that AADAC is expressed in dog intestine, the mRNA expression levels of dog AADAC in the intestine and liver were initially measured with real-time reverse-transcription polymerase chain reaction. The mRNA expression was detected in both the liver and intestine, and the level in the liver was 2.7-fold higher than that in the intestine (data not shown). Because protein expression is not necessarily correlated with mRNA expression, we attempted to evaluate the expression of AADAC protein in DIMs and DLMs by immunoblot analysis. However, a commercially available polyclonal antibody against human AADAC did not react with dog AADAC, even though it reacted with rat and mice AADAC (data not shown). Accordingly, we sought to alternatively investigate dog AADAC expression by measuring the hydrolase activity of a specific substrate of human AADAC. Because recombinant dog AADAC could not efficiently hydrolyze indiplon, phenacetin was used as a marker substrate of AADAC for the dog study (Fig. 6A). Such species differences are not surprising because it has been previously reported that there are species differences in substrate specificity among human, rat, and mouse AADAC (Kobayashi et al., 2012b).
Phenacetin hydrolase activity was detected in both DIMs and DLMs, although the activity in DIMs was only 8% of that in DLMs. Therefore, it would be permissible to claim that AADAC was functionally expressed in both dog intestine and liver, although the expression level in the intestine was relatively low. The expressions of CES1 and CES2 in DIMs and DLMs were also investigated by measuring fenofibrate and procaine hydrolysis, and it was found that both enzymes are expressed in DLMs but not in DIMs (Fig. 6, B and C). This result was consistent with a previous report showing no expression of CES1 and CES2 in dog intestine (Taketani et al., 2007). Collectively, it was demonstrated that AADAC, but not CES enzymes, is expressed in dog intestine.
The Km values of recombinant dog AADAC and DIMs for prasugrel hydrolysis showed similar values (Table 3). In addition, inhibition profiles of recombinant dog AADAC and DIMs were similar (Fig. 7B). The activity in DIMs (Vmax, 29.8 ± 2.0 nmol/min/mg) was much lower than that in DLMs (Vmax, 1326.2 ± 107.2 nmol/min/mg) (data not shown), and the tendency was similar to the finding for phenacetin hydrolysis (Fig. 6A). These results suggest that AADAC majorly contributes to prasugrel hydrolysis in dog intestine. The inhibition potencies of DFP and eserine to prasugrel hydrolysis by dog AADAC appeared to be slightly lower than those by human AADAC. It was reported that the IC50 value of loperamide was 0.562 μM for purified human CES2, whereas that for purified dog CES2 was 93.6 μM (Williams et al., 2011). Thus, the differences in the inhibitory potencies of DFP and eserine are due to species differences.
The CLint value of prasugrel hydrolase activity in dog intestine was 20-fold lower than that in human intestine (Tables 1 and 3). The difference may be explained by the difference in the expression levels of CES2 and AADAC between human and dog intestines. CES2 is highly expressed in human intestine but not in dog intestine. Because phenacetin hydrolase activity in DIMs (322.5 ± 65.1 pmol/min/mg protein at 5 mM phenacetin) was lower than that in HIMs (1927.5 ± 67.9 pmol/min/mg protein, data not shown), it was surmised that AADAC expression in dog intestine would be much lower than that in human intestine. Nevertheless, it has been reported that prasugrel is extensively hydrolyzed in the small intestine after oral doses in dogs (Hagihara et al., 2011). This would be due to the high absolute CLint value of prasugrel hydrolysis in DIMs (5.6 ± 0.3 ml/min/mg protein).
In a recent study, the inhibitory potencies of 542 compounds against human AADAC using a recombinant enzyme was investigated, and it was found that several compounds or drugs, such as benzbromarone and vinblastine, potently inhibited AADAC activity (Shimizu et al., 2014b). However, there are no clinical reports for drug-drug interaction in which the involvement of AADAC is suspected, probably because such a combination would be rare in clinical studies. As for prasugrel, a drug-drug interaction caused by metabolic inhibition has not been reported. One feasible reason would be that clearance of prasugrel hydrolysis in both the intestine and liver is much higher. If drugs that are specifically hydrolyzed by AADAC with low Km values are found or developed, we would have to pay attention to the possibility of drug-drug interaction.
In conclusion, we found that prasugrel hydrolysis is catalyzed not only by CES enzymes but also by AADAC in human. AADAC majorly contributes to prasugrel hydrolysis in dog intestine, where CES1 and CES2 are not expressed. Thus, it should be noted that AADAC is an important esterase to be considered in drug development.
Authorship Contributions
Participated in research design: Kurokawa, Fukami, Nakajima.
Conducted experiments: Kurokawa, Fukami, Yoshida.
Contributed new reagents or analytic tools: Kurokawa, Fukami.
Performed data analysis: Kurokawa, Fukami.
Wrote or contributed to the writing of the manuscript: Kurokawa, Fukami, Nakajima.
Footnotes
- Received November 4, 2015.
- Accepted December 29, 2015.
This work was supported in part by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science [Grant 26860098].
Abbreviations
- AADAC
- arylacetamide deacetylase
- CES
- carboxylesterase
- CYP
- cytochrome P450
- DFP
- diisopropyl fluorophosphate
- DIM
- dog intestinal microsome
- DLM
- dog liver microsome
- DMSO
- dimethylsulfoxide
- HIM
- human intestinal microsome
- HLM
- human liver microsome
- HPLC
- high-performance liquid chromatography
- PMSF
- phenylmethylsulfonyl fluoride
- RAF
- relative activity factor
- Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics