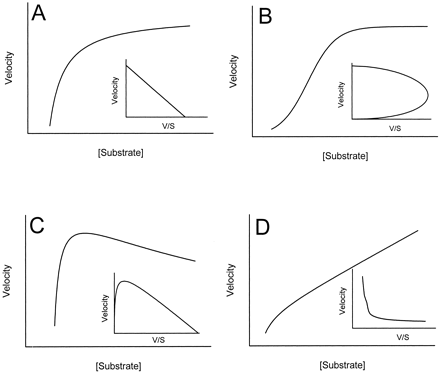
The cytochrome P450 enzymes are involved in the biotransformation of both xenobiotic and endobiotic hydrophobic compounds, implicated in the bioactivation of certain procarcinogens (e.g., benzo[a]pyrene), and responsible for many metabolism-based drug-drug interactions (Wrighton and Stevens, 1992). Consequently, the goal of drug metabolism and toxicology labs is to not only to determine the P450 isoform contribution to the metabolism of a given compound but also to understand the various factors that effect the activity and behavior of these isoforms. Classically, metabolism of a particular compound is described kinetically using the Michaelis-Menten equation, which yields a hyperbolic rate profile (Fig.1A) and estimates of maximal reaction velocity (Vmax) and apparentKm. It is evident, however, that for some drugs the kinetic profile is better described by a non-Michaelis-Menten or atypical kinetic model.
Representative kinetic plots showing Michaelis-Menten hyperbolic kinetic profile (A), sigmoidal autoactivation profile (B), substrate inhibition profile (C), and biphasic kinetic profile (D).
Insets show corresponding Eadie-Hofstee plots for each kinetic profile.
Atypical kinetic profiles are proposed to fall into five categories: activation, autoactivation, substrate inhibition, partial inhibition, and biphasic metabolism (Korzekwa et al., 1998). Activation occurs when P450 enzyme activity for a substrate is increased in the presence of another compound, due to structural or electronic changes of the enzyme, and is often referred to as heterotropic positive cooperativity (the activator is different from the substrate). Autoactivation (homotropic positive cooperativity) occurs when a compound activates its own metabolism, resulting in a sigmoidal kinetic profile (Fig. 1B). Kinetic parameters from sigmoidal data are often estimated using the Hill equation, with the Hill coefficient describing the degree of sigmoidicity (n > 1). Substrate inhibition occurs when the reaction velocity of product formation decreases once a particular substrate concentration is surpassed, resulting in a convex reaction profile (Fig. 1C). Partial inhibition is when incomplete inhibition is observed even in the presence of saturating concentrations of a second substrate/inhibitor. The fifth atypical kinetic phenomenon reported is a biphasic saturation profile (Fig. 1D), indicative of an enzyme with both a low- and high-affinity binding component. This is similar to a dual-enzyme model but is interpreted as a single enzyme with multiple binding regions (Korzekwa et al., 1998).
Previously, atypical kinetic profiles were most likely misinterpreted, citing assay or experimental variability, or not observed, perhaps due to lack of sensitive analytical techniques or the paucity of data points collected, particularly at the low end of the substrate concentration range. However, with today's improved analytical technology, scientists in drug metabolism are capable of designing more complete kinetic studies and thus are becoming more cognizant of atypical kinetic profiles. This is reflected by the increase in examples reported in the literature over the past few years. This review will highlight some specific examples of atypical kinetics, as well as the proposed mechanisms of some of these interactions and the implications of atypical kinetics with respect to in vitro-in vivo correlations. In addition, artifactual sources of atypical kinetic profiles and in vivo observations will be discussed.
Atypical Kinetic Profiles
Activation (Heterotropic Positive Cooperativity).
Activation of drug metabolism was first observed many years ago in microsomes from rat tissue, as 7,8-benzoflavone (α-naphthoflavone) was shown to activate the hydroxylation of benzo[a]pyrene in control and phenobarbital-treated rats (Wiebel et al., 1971). Several other studies undertaken shortly thereafter concurred with these findings (Wiebel and Gelboin, 1975; Kapitulnik et al., 1977;Huang et al., 1981), but the particular isoforms involved in this metabolism and activation had not been isolated or identified. Later studies established that purified CYP3A enzymes are directly activated by 7,8-benzoflavone (Shou et al., 1994). Schwab and colleagues (1988)concluded that both progesterone 6β-hydroxylation and 17β-estradiol 2-hydroxylation are activated by 7,8-benzoflavone in humans and rabbits, based on the observation that the apparentKm of these reactions decreased andVmax increased in the presence of 7,8-benzoflavone. Similarly, Shou and coworkers (1994) demonstrated that 7,8-benzoflavone activates phenanthrene metabolism by increasing the Vmax, although the apparentKm was unaffected. Also discovered was that 7,8-benzoflavone is a substrate for CYP3A4, as phenanthrene was found to decrease the Vmax of 7,8-benzoflavone metabolism without affecting the apparentKm. This particular finding generated ideas as to the mechanism of activation (discussed in a later section).
Because of the numerous studies showing 7,8-benzoflavone activation of CYP3A4 (Table 1), it has been labeled as the classic CYP3A4 activator. However, several groups have observed that compounds other than 7,8-benzoflavone may activate CYP3A4 activity. Maenpaa et al. (1998) have shown that testosterone stimulates the formation of 4-hydroxymidazolam from midazolam in liver microsomes and expressed CYP3A4, whereas Kenworthy et al. (2001) have shown that testosterone also activates the formation of 3-hydroxydiazepam andN-desmethyldiazepam from diazepam by 374 and 205%, respectively. Meanwhile, Ludwig et al. (1999) observed that meloxicam metabolism is activated by quinidine in a dose-dependent manner. Interestingly, quinidine also activates the metabolism of diclofenac to its 5-hydroxy derivative and warfarin to its 4′- and 10-hydroxy metabolites in human liver microsomes, recombinant CYP3A4, and in human hepatocyte suspensions, indicating that this interaction occurs in intact cells and is probably not an in vitro artifact (Ngui et al., 2000, 2001). These observations are not altogether surprising considering the number of substrates with varying sizes and molecular weights that CYP3A4 accommodates. In fact, Wang et al. (2000) suggest that these types of interactions with CYP3A4 are substrate-dependent, implying that perhaps the interactions driving these atypical kinetic observations depend, at least partially, upon a compound's physical and chemical properties. An interesting observation from this study was that 50 to 100 μM testosterone stimulated the formation of 4-hydroxy midazolam but inhibited 1′-hydroxylation, suggesting an alteration of regiospecificity, perhaps due to the presence of testosterone in the active site. In addition, testosterone did not interact at all with nifedipine metabolism but partially inhibited terfenadinet-butyl-hydroxylation (Wang et al., 2000) (see Partial Inhibition for further discussion of substrate-dependent interactions).
Examples of activation kinetics (heterotropic positive cooperativity) reported in the literature in various in vitro systems
Just as compounds other than 7,8-benzoflavone can activate CYP3A4, the activity of other isoforms may be stimulated by the presence of certain compounds. For example, Korzekwa et al. (1998) and Hutzler et al. (2001b) have shown that dapsone activates CYP2C9-mediated metabolism of a number of compounds, including flurbiprofen, naproxen, and piroxicam, albeit to differing degrees, further supporting the contention that these interactions may be substrate-dependent. Furthermore, Korzekwa et al. (1998) demonstrated that dapsone is also metabolized by CYP2C9, analogous to 7,8-benzoflavone metabolism by CYP3A4. Thus, there seems to be some possible mechanistic similarities for activation between these isoforms. Table 1 shows some of the examples of activation that have been observed.
Autoactivation (Homotropic Positive Cooperativity).
With regard to autoactivation, according to an article by Ekins et al. (1998a), the most frequently reported CYP3A4 substrates that display autoactivation kinetics include aflatoxin B1, amitriptyline, and progesterone, although some other compounds displaying autoactivation kinetics have been reported (Table 2). In addition to CYP3A4, it has been demonstrated that Escherichia coli- and RECO-expressed CYP1A2 show autoactivation kinetics for ethoxyresorufin O-deethylation (Ekins et al., 1998a). However, ethoxyresorufin O-deethylation in human liver microsomes and insect-expressed sources of CYP1A2 showed classical Michaelis-Menten kinetics, suggesting that atypical kinetic profiles may not only be substrate-dependent but also enzyme source-dependent. One other P450 that displays autoactivation kinetics is CYP2B6; Ekins et al. (1998b) observed that CYP2B6-mediated testosterone 16β-hydroxylation metabolism data are best fit to the Hill equation (n = 1.3). Autoactivation of CYP3A has also been observed in rat hepatocytes, as noted by the sigmoidal nature of the formation curves for methoxymorphinan and dextrorphan from dextromethorphan, suggesting that this is not solely a microsomal phenomenon (Witherow and Houston, 1999). More recent evidence suggests that phase II enzymes can display autoactivation kinetics also. In a study by Fisher et al. (2000), kinetic profiles of estradiol-3-glucuronide and acetaminophen-O-glucuronide were best fit to the Hill equation, as Eadie-Hofstee plots of the data were indicative of autoactivation (see Fig. 1B). This observation is the first article involving autoactivation of glucuronosyltransferases in microsomal incubations, although bilirubin conjugation by UGT1A1 in hepatocytes has been shown to display autoactivation kinetics also (Bruni and Chang, 1999). These data suggest that atypical kinetics may not be limited to phase I metabolic reactions.
Examples of autoactivation kinetics (homotropic positive cooperativity)
Substrate Inhibition.
Substrate inhibition is another atypical kinetic phenomenon that has been observed in in vitro studies. Although the mechanism of substrate inhibition has yet to be fully determined, it has been described by a two-site model in which one binding site is productive, whereas the other site is inhibitory and operable at high substrate concentrations, resulting in decreased velocity with increasing concentrations (Shou et al., 2001b). One early study showed that tenoxicam 5′-hydroxylation by the CYP2C family strongly inhibits its own metabolism at concentrations higher than 100 to 150 μM (Zhao et al., 1992). Interestingly, our lab has demonstrated that piroxicam 5′-hydroxylation also demonstrates substrate inhibition in both human liver microsomes and purified baculovirus-expressed CYP2C9 but not in expressed CYP2C9 microsomes (Tracy et al., 2002). Yet another CYP2C9 substrate displaying substrate inhibition kinetics is the new cyclooxygenase 2-selective nonsteroidal anti-inflammatory drug celecoxib (Tang et al., 2000). Furthermore, Spracklin et al. (1997) have observed that halothane metabolism by CYP2E1 to trifluoroacetic acid and bromide displays substrate inhibition, one of the few examples of atypical kinetics with CYP2E1. Concerning CYP3A4, Schrag and Wienkers (2001)observed that triazolam 1′-hydroxylation displayed substrate inhibition kinetics at higher concentrations, confirmed by an Eadie-Hofstee plot, which showed a hook in the upper quadrant (Fig. 1C) indicative of substrate inhibition. Most recently, Lin et al. (2001) have observed substrate inhibition kinetics for several compounds that are routinely used as probes for particular P450 isoforms. For example, CYP1A2-catalyzed ethoxyresorufin metabolism to resorufin, CYP2D6-catalyzed dextromethorphan metabolism to dextrorphan, and testosterone and progesterone metabolism by CYP3A4 all displayed substrate inhibition kinetics (Lin et al., 2001). The data for ethoxyresorufin are particularly interesting, considering the work done by Ekins and coworkers (1998a) suggesting autoactivation of ethoxyresorufin metabolism not substrate inhibition. At this time, the incongruence of these findings is not readily explained, although as stated earlier, enzyme source and interlab variability may be factors. Several examples of substrate inhibition are shown in Table3.
Substrates reported to display substrate inhibition kinetics in various in vitro systems
Partial Inhibition.
Partial inhibition occurs when a compound, acting as either a competing substrate or inhibitor for a particular P450 isoform, incompletely inhibits another substrate's metabolism, even at saturating concentrations. This phenomenon has been further explained by suggesting that both the substrate and inhibitor may have access to the reactive oxygen within the active site, forming a substrate-inhibitor-enzyme complex that is still productive (Shou et al., 2001b). It is no surprise then that partial inhibition is often observed with CYP3A4 since this enzyme has been shown to accommodate multiple substrates simultaneously. While studying the simultaneous metabolism of testosterone and erythromycin by CYP3A4, Wang et al. (1997) observed that the inhibition mechanism for this interaction was not purely competitive. At a fixed concentration of 60 μM testosterone, the rate of 6β-hydroxylation was greatly reduced by high concentrations of erythromycin, suggesting competitive inhibition between substrates. However, at higher fixed amounts of testosterone (250 and 500 μM), only partial inhibition of 6β-hydroxylation was observed (Wang et al., 1997). Wang and coworkers (2000) have also observed partial inhibition of CYP3A4-mediated testosterone metabolism by midazolam and terfenadine. In a similar study, Korzekwa et al. (1998) observed that phenanthrene displayed partial inhibition of 7,8-benzoflavone metabolism, while not affecting its apparentKm, suggesting that 7,8-benzoflavone was not displaced from the active site and that both compounds were binding in distinct regions within the active site.
The presence of multiple binding regions within a single enzyme active site, which probably contributes to substrate-dependent effects, is a potential cause of partial inhibition and may complicate accurate determination of Ki values for certain compounds. This substrate dependence in the determination ofKi values has been most frequently noted for CYP3A4. In addition to the work of Wang and colleagues (2000)(see Activation), others have observed the need to use multiple probes when assessing inhibitory constants for various potential inhibitors of CYP3A4 (Kenworthy et al., 1999; Stresser et al., 2000; Lu et al., 2001). This probably means thatKi values for inhibitors of enzymes displaying atypical kinetics (multiple binding regions) may be different depending upon which probe substrate is being used.
Biphasic Kinetics.
A biphasic kinetic profile is characterized by an initial Michaelis-Menten-like increase in velocity with increasing substrate concentration. However, the profile does not become asymptotic, with the reaction profile eventually becoming linear with increasing substrate concentration (Fig. 1D). This results in the inability to predict an apparent Vmax and thus apparent Km. Some examples include naproxen demethylation by CYP2C9 in which saturation is unobtainable up to 1800 μM (Korzekwa et al., 1998; Hutzler et al., 2001b), and CYP3A4-mediated naphthalene metabolism to 1-naphthol (Korzekwa et al., 1998). More recent evidence for biphasic kinetics include 7-ethoxycoumarin O-deethylation and aminopyrineN-demethylation in recombinant yeast microsomes expressing rat CYP1A1 and yeast NADPH-P450 reductase (Inouye et al., 2000). This is the first article showing atypical kinetics of P4501A1-mediated 7-ethoxycoumarin metabolism, an often used probe for CYP1A1 activity. In addition, Oda and Kharasch (2001) discovered that levo-α-acetylmethadol (LAAM) demethylation and nor-LAAM demethylation to dinor-LAAM exhibit biphasic Eadie-Hofstee plots in cDNA-expressed CYP3A4 enzymes. Most recently, it was discovered that the rates of 4-nitrophenol and formaldehyde production from theO-demethylation of 1-methoxy-4-nitrobenzene by CYP1A2 isolated from rabbit liver produced biphasic plots (Miller and Guengerich, 2001). As suggested by the above studies, examples of biphasic kinetics are becoming more prevalent, with several P450 isoforms apparently exhibiting this type of behavior (Table4).
Substrates reported to display biphasic kinetics in various in vitro systems
Mechanistic Models of Atypical Kinetics
Since the first observation of 7,8-benzoflavone activating the metabolism of benzo[a]pyrene, much effort has been put forth to understand the mechanism of this and other atypical kinetic events. Shou et al. (1994) first suggested that both substrate and activator may be simultaneously present in the active site, both having access to the reactive oxygen. This conclusion was based on kinetic evidence that 7,8-benzoflavone increased theVmax of phenanthrene metabolism without changing the apparent Km. Conversely, phenanthrene decreased theVmax of 7,8-benzoflavone metabolism without increasing the apparent Km, suggesting that neither compound displaces the other from the active site, resulting in near-simultaneous metabolism. This study provided the first indirect evidence that more than one molecule could be present in and influence the active site. In a related article, perhaps the most comprehensive work examining atypical kinetics, Korzekwa et al. (1998) provided further evidence for the theory of multiple binding regions within the active site. This group suggested that a two-site binding model might be used to describe all atypical kinetic observations, including those observed in isoforms other than CYP3A4. Korzekwa and coworkers also demonstrated several of the theoretical saturation profiles for an enzyme showing sigmoidal, biphasic, and substrate inhibition kinetics, comparing the apparentKm andVmax parameters of the two proposed binding sites. One of the most interesting observations from this study was the biphasic metabolism of naproxen by CYP2C9 and naphthalene by CYP3A4. The authors suggest that this profile may also be explained by a two-site binding model, one site having a low affinity (apparentKm) and low capacity (Vmax), the other having high affinity and high capacity. This type of profile is similar to that of a two-enzyme model, except that the data are derived from a single purified or expressed enzyme. Hutzler et al. (2001b) expanded on this work and observed that in the presence of 100 μM dapsone, a substrate and activator for CYP2C9, the naproxen demethylation kinetic profile was converted from biphasic to hyperbolic, as evidenced by linearization of the Eadie-Hofstee plots. This may be rationalized by the proposal that dapsone binds in the region responsible for the high-apparent Km component of naproxen metabolism, rendering only one naproxen binding site operable for naproxen demethylation, resulting in a typical hyperbolic saturation profile. In addition, these same researchers observed near-simultaneous metabolism of flurbiprofen and dapsone, with the presence of flurbiprofen minimally affecting dapsone N-hydroxylation, further suggesting that CYP2C9 may have some similar characteristics to CYP3A4 (i.e., multiple binding regions).
Site-directed mutagenesis studies by Harlow and Halpert (1998)have added compelling structural evidence supporting the multisite hypothesis. Their group constructed a L211F/D214E double mutant of CYP3A4, which was designed to mimic the action of effector (7,8-benzoflavone) by reducing the size of the active site with the bulkier phenylalanine and glutamate residues. Results showed that the double mutant exhibited an increased rate of testosterone and progesterone 6β-hydroxylation and a lower level of activation by 7,8-benzoflavone, suggesting that the active site was the most likely location of activator (effector) binding. In addition, studies have shown that residues 301, 304, 305, and 309, predicted to be within substrate recognition site 4 of CYP3A4, are also involved in steroid and effector binding (Domanski et al., 1998, 2000). Other studies supporting the multisite hypothesis include those of Ngui et al. (2000)and Schrag and Wienkers (2001). Briefly, Ngui and coworkers observed that quinidine stimulated diclofenac metabolism while maintaining normal access to the ferriheme-oxygen complex, whereas Schrag and Wienkers observed an alteration of the metabolite ratio (4-hydroxy triazolam/1′-hydroxy triazolam) for triazolam due to the presence of testosterone, referred to as differential kinetics (when an effector activates the metabolism of a given substrate at one position but inhibits metabolism at a different position). In addition, Hosea et al. (2000) provide evidence for multiple binding sites for CYP3A4 through spectral binding studies with a number of CYP3A4 substrates and a series of nonmetabolized peptide ligands, whereas Szklarz and Halpert (1998) have generated a three-dimensional homology model of CYP3A4, which shows the ability to dock multiple substrates or the substrate and effector within the active site simultaneously.
In agreement with Korzekwa et al. (1998), Lin and colleagues (2001) suggest that substrate inhibition may be explained by a two-site binding model in which the two sites may either be neighboring or distant from each other within the active site. They hypothesize that in the case of substrate inhibition, one site is favorable for oxidation, whereas the other site is nonproductive, which differs from the theory that both sites may have access to the reactive oxygen. This is further explained by suggesting that when a substrate binds to the inhibitory site (at high concentrations), the complex (substrate-enzyme-substrate) is less capable of forming product than the enzyme-substrate complex. A scheme representing the possible enzyme-substrate-effector interactions and product formation has been presented in a recent article by Shou et al. (2001b). Nonetheless, whether one or the other or both sites are productive will probably depend on the physical and chemical properties of the compounds in question and/or the conformational dynamics of the enzyme.
Although most studies show evidence for a multisite binding model, other possibilities, such as the presence of an allosteric site, have not been completely disproven. Ueng et al. (1997) suggested an allosteric model for CYP3A4, although the proximity of the allosteric site relative to the active site is not defined. Another study hypothesized that multiple conformers of CYP3A4 exist because of kinetic studies showing different rates of carbon monoxide binding in the presence of a number of CYP3A4 substrates (Koley et al., 1995). This group later suggested that activation of benzo[a]pyrene metabolism by 7,8-benzoflavone resulted from an allosteric mechanism in which the presence of 7,8-benzoflavone converts an nonproductive enzyme conformation pool to a productive one, resulting in increased levels of benzo[a]pyrene metabolite (Koley et al., 1997). One of the most intriguing explanations for the cause of atypical kinetics is one in which the multiple binding-site model and a conformationally based model are combined, as suggested byAtkins et al. (2001). The reason for this suggestion is that multiple conformations alone cannot account for sigmoidal velocity versus substrate concentration curves, whereas the multiple binding-site theory does not fully explain the lack of reciprocal effects of certain drug interactions. Therefore, a scheme is proposed in which two enzyme conformers are in equilibrium, and each conformer can bind two substrates at a single active site. Another possibility suggested byEkins et al. (1998a) is the contribution of water molecules in the active site and hydrogen bonding. They theorized that the displacement of water molecules by a second substrate or effector might cause fluctuations in the conformation of the enzyme, perhaps leading to changes in active-site characteristics and enhanced catalytic activity. In addition, it has been suggested that displacement of water molecules from the active site by activators may result in decreased uncoupling of the P450 reaction cycle, resulting in an apparent increased enzyme activity (Shou et al., 1994).
The interaction between P450 and cytochromeb5 and oxidoreductase may also be involved in the production of atypical kinetic profiles. It is possible that effector binding increases or enhances the interaction of P450 with either or both cytochrome b5 and oxidoreductase. However, Ngui et al. (2000) were able to exclude the role of cytochrome b5 in the stimulation of diclofenac metabolism by quinidine because the effect of quinidine was maximal in the absence of cytochromeb5. In addition, Ngui and coworkers observed no change in cytochrome c reduction by oxidoreductase in the presence of quinidine. Similarly, our lab has observed that flurbiprofen 4′-hydroxylation is activated by dapsone despite the absence of cytochrome b5(T.S.T. and J.M.H., unpublished observations). However, in a study byLee et al. (1997) using rat liver microsomes, it was suggested that the mechanism of cytochrome P450 activation involves electron transfer steps, probably from cytochrome b5. This was based on the observation that the formation ofN-acetyl-p-benzoquinone imine from acetaminophen was not stimulated by 5 mM caffeine or 50 μM 7,8-benzoflavone in liver microsomes when supported with cumene hydroperoxide via the peroxide shunt, whereas approximately 3-fold activation was observed for both caffeine and 7,8-benzoflavone in NADPH-supported incubations (Lee et al., 1997). In addition, Lee and coworkers observed that an inhibitory cytochromeb5 antibody significantly diminished the activation ofN-acetyl-p-benzoquinone imine formation by caffeine but not 7,8-benzoflavone, suggesting different mechanisms of activation by these compounds. Interestingly, Yamazaki et al. (1996)observed that apo-b5 (without heme) was as effective as holo-b5 (with heme) in stimulating testosterone 6β-hydroxylation and nifedipine oxidation in a system containing NADPH-P450 reductase, suggesting thatb5 may activate CYP3A4 by inducing a conformational change in the enzyme and not by increasing electron flow. However, it has recently been suggested that apo-b5 may be converted to holo-b5 by a heme transfer reaction from the CYP3A4 preparation, which may explain stimulation of CYP3A4 activity (Guryev et al., 2001).
Although much evidence exists for the preceding mechanisms of atypical kinetics, no one theory has been proven conclusively. It is possible that one of the above-mentioned mechanisms, a combination of mechanisms, or some as yet undetermined mechanism(s) is probably operable and contributing to the atypical kinetic profiles of many drugs.
Kinetic Modeling of Atypical Kinetics
Along with observing atypical kinetic profiles comes the task of determining how to properly model the data to generate accurate kinetic parameter estimates. As mentioned previously, atypical kinetic profiles have probably been overlooked or ignored in the past. However, it is now apparent that ignoring or truncating nonhyperbolic data can lead to erroneous kinetic parameter estimates, as standard Michaelis-Menten hyperbolic curves are forced through the data rather than using a more appropriate kinetic model. For example, it has been suggested that substantial underestimation of Vmaxmay occur when substrate inhibition is observed if the high-concentration data points are ignored (Houston and Kenworthy, 2000). This was validated by Lin and coworkers (2001), who observed that the estimated Vmax values for several reactions showing substrate inhibition were 0.91- to 1.8-fold lower when fitted to the Michaelis-Menten equation rather than a more appropriate substrate inhibition equation. Likewise, Houston and Kenworthy (2000) suggest that for a sigmoidal kinetic profile, either underestimation or overestimation of intrinsic clearance may occur if a hyperbolic curve is forced through the data, with the apparentKm(S50) term most likely affected. Therefore, it is imperative that the proper kinetic model (e.g., substrate inhibition, biphasic, or sigmoidal) be used to estimate kinetic parameters from the data.
With respect to the equations used for modeling atypical kinetic data, most have been derived to describe the presence of multiple binding sites within the active site. Comprehensive discussions of the modeling of atypical kinetic data and the equations typically used can be found in several articles (Korzekwa et al., 1998; Shou et al., 1999; Houston and Kenworthy, 2000; Kenworthy et al., 2001; Shou et al., 2001a), with each showing enzyme-substrate-effector kinetic schemes from which these equations are generated. However, one must realize that when applying these more complex models to atypical kinetic data, there may be several solutions that satisfactorily model the same set of data. Consequently, a good fit to a model derived from a particular kinetic scheme does not ensure an accurate description of the enzyme-substrate-effector interactions that have occurred.
Artifactual Sources of Atypical Kinetics
Although the presence of atypical kinetics seems to be real, it is important to perform experiments in a fashion so as to eliminate any artifactual causes of atypical kinetic profiles. Several examples of artifactual sources of sigmoidicity and substrate inhibition have been mentioned by Houston and Kenworthy (2000). Examples cited include significant substrate depletion, nonspecific binding of substrate to the incubation matrix, cellular active transporter systems, lack of analytical sensitivity, low substrate solubility, and use of multienzyme systems (e.g., human liver microsomes). Significant substrate depletion results in deviations from initial rate conditions and leads to erroneous estimation of kinetic parameters even in the absence of atypical kinetics. As a result, it is important to monitor substrate consumption and even effector consumption if the effector happens to be metabolized as well. Nonspecific binding to microsomal proteins has been shown to be another potential problem in in vitro studies (Obach, 1997; Venkatakrishnan et al., 2000). One such example is the observation that addition of 750 μg/ml of inactive control microsomes produced a 1.8-fold increase in the apparentS50 for amitriptyline metabolism, although no effect on the Vmax or Hill coefficient was observed (Venkatakrishnan et al., 2000). As a result, it may be beneficial to run the experiment at the lowest possible protein concentration to help reduce the effects of nonspecific binding. With respect to low analytical sensitivity and solubility issues, these cause a lack of confidence in quantitation or “effective” concentration at the low and high end of the substrate concentration range, respectively. In addition, Houston and Kenworthy (2000) suggest obtaining a minimum of 10 data points before concluding the presence of atypical kinetic profiles. Meanwhile, Shou et al. (1999) suggest obtaining upwards of 20 to 30 data points, especially around the inflection points, to better define the fitted curve. In addition, caution should be used when describing atypical kinetic phenomena derived from experiments using multienzyme systems, such as human liver microsomes. The atypical kinetic observations derived from these systems may occur simply as a consequence of the combined actions of more than one enzyme rather than the atypical behavior of a single enzyme. Thus, conclusions must also be verified using single enzyme sources (e.g., expressed/purified enzymes).
Other considerations when conducting these types of studies may be salt, cytochrome b5, and organic solvent concentrations in the incubation. Schrag and Wienkers (2000)have observed that pyrene metabolism by CYP3A4 is sigmoidal in the absence of magnesium but biphasic in the presence of 10 mM magnesium. In this work, evidence suggested a change in CYP3A4-active site conformation in the presence of magnesium, resulting in the different kinetic profile. In a similar study, Maenpaa et al. (1998) observed that the addition of 5 and 50 mM MgCl2 to microsomal incubations resulted in minimal changes in midazolam 1′-hydroxy and 4-hydroxy metabolites, whereas the addition of 150 mM MgCl2 resulted in substantial inhibition of both metabolites. Meanwhile, 1′-hydroxy and 4-hydroxy midazolam formation was completely inhibited by 30 mM CaCl2in microsomal incubations. In another study, acetonitrile and acetone were found to activate the NADPH-dependent tolbutamide hydroxylation nearly 3-fold in human liver microsomes and CYP2C9-reconstituted system when incubated at 2 to 4% final organic solvent concentrations (Palamanda et al., 2000). From these examples, it is evident that investigators must remain cognizant of the many different conditions that may be artifactual sources of atypical kinetics. In addition, the above list is probably not all-inclusive, and thus, there may be other confounding factors also involved in the observation of atypical kinetics.
In Vivo Considerations
Although it is known that atypical kinetics exist in vitro, an important question is whether they also occur in vivo since they may have considerable pharmacological or toxicological implications. However, according to Houston and Kenworthy (2000), whether these types of interactions occur in vivo and whether they are clinically relevant is not the primary issue. Even if these interactions are not observed in vivo, their presence in vitro will affect how data are scaled to the in vivo situation. It is best to fully describe the in vitro data and then abstract the useful parameter(s) for extrapolation (Houston and Kenworthy, 2000). Nonetheless, in vivo studies designed to determine the presence of atypical kinetics would be beneficial, but only a few have actually been performed. The first examples of activation of drug metabolism in vivo were studies by Lasker et al. (1982, 1984), who observed that the i.p. administration of flavone, nobiletin, tangeretin, or 7,8-benzoflavone concurrently with zoxazolamine immediately stimulated the total body metabolism of zoxazolamine to its 6-hydroxy metabolite in neonatal rats in a time course consistent with activation. This activation was found to be dose- and dosing time-dependent because little activation of zoxazolamine metabolism was observed with a low dose of zoxazolamine and when the activator was administered 2 to 4 h before zoxazolamine (Lasker et al., 1984). It is noteworthy that Lasker and coworkers explored the possibility that the flavone compounds could activate zoxazolamine metabolism by displacing it from binding sites on plasma protein or from binding sites in the total body homogenates by performing equilibrium dialysis studies. Results showed no difference in zoxazolamine binding in the presence of flavone. Based on this observation, it was determined that this activation in rats was probably P450-mediated and thus the first noted example of in vivo activation of drug metabolism.
More recently, it has been discovered that activation of P450-mediated drug metabolism may be observed in vivo in monkeys. In previous studies, Tang et al. (1999b) observed that the formation of diclofenac metabolites via CYP3A4 increased more than 4-fold in human liver microsomes in the presence of quinidine. Since similar results were seen in monkey liver microsomes, Tang and coworkers (1999a) followed this work with an in vivo study in monkeys and observed that in the presence of quinidine, diclofenac clearance was enhanced in three male rhesus monkeys by 57, 56, and 56%, respectively. The plasma protein binding and blood/plasma ratio of diclofenac remained unchanged in the presence of quinidine, and plasma samples were taken in a time frame too short for the de novo synthesis of P450 proteins. This suggested that the interaction was P450-mediated and the reduction in diclofenac levels compared with control was not due to induction (1999a).
To date, only one human in vivo study has specifically addressed the issue of atypical kinetics with respect to enzyme activation. Based on the in vitro observation of activation of CYP2C9-mediated flurbiprofen hydroxylation by dapsone, we conducted a clinical study to assess the in vivo relevance of this interaction (Hutzler et al., 2001a). Results from this in vivo study suggest that activation of flurbiprofen metabolism by dapsone occurs to a minimal extent in humans, with only about a 10% increase in flurbiprofen apparent oral clearance after 7 days of dapsone dosing. This is much less than the activation observed in purified enzyme (∼100%) and human liver microsomes (∼40%) (Korzekwa et al., 1998). Reasons for the discrepancy of the in vivo observations to in vitro results are unclear at this time, but ideas such as reduced effective drug concentration due to protein binding and competing metabolic pathways have been hypothesized (Hutzler et al., 2001a). It is apparent that more extensive studies need to be pursued to determine whether observed in vitro atypical kinetic interactions may also occur in vivo and to eliminate alternative explanations that may contribute to these kinetic observations.
Conclusions
With the presence of atypical kinetics in P450-mediated drug metabolism seemingly on the rise and the lingering potential for drug-drug interactions, it is imperative that kinetic studies are carefully designed and the data correctly interpreted. This is best accomplished through the use of appropriate kinetic models to estimate the parameters that describe these interactions and to avoid artifactual sources of atypical kinetics. Forcing Michaelis-Menten curves through nonhyperbolic data may lead to erroneous estimations and, thus, poor correlations from in vitro to in vivo situations. In addition, most examples of atypical kinetics have been explained by a multiple binding-site model. The presence of multiple binding sites, in turn, may affect the accurate Kidetermination for potential inhibitors. However, until more conclusive evidence of multiple occupancy in the active site is generated, other possibilities cannot be discounted. Lastly, more studies need to be conducted to determine whether examples of atypical kinetics are relevant in vivo or merely an in vitro artifact. Nonetheless, it is important to understand atypical kinetics in vitro because they effect in vitro-in vivo correlations of drug metabolism.
Footnotes
- Received October 1, 2001.
- Accepted December 21, 2001.
- The American Society for Pharmacology and Experimental Therapeutics
References
 J. Matthew Hutzler received a Bachelor's degree in biology in 1996 from Shepherd College (Shepherdstown, WV), where he was named the Outstanding Senior in Biology and a McMurran Scholar. He recently received the Ph.D. degree from the Department of Basic Pharmaceutical Sciences at West Virginia University (Morgantown, WV) working with Dr. Timothy S. Tracy in the area of drug metabolism. His research dissertation involved studying factors that affect the regulation of cytochrome P450 2C9 activity, in particular, the activation of CYP2C9 activity by dapsone.
J. Matthew Hutzler received a Bachelor's degree in biology in 1996 from Shepherd College (Shepherdstown, WV), where he was named the Outstanding Senior in Biology and a McMurran Scholar. He recently received the Ph.D. degree from the Department of Basic Pharmaceutical Sciences at West Virginia University (Morgantown, WV) working with Dr. Timothy S. Tracy in the area of drug metabolism. His research dissertation involved studying factors that affect the regulation of cytochrome P450 2C9 activity, in particular, the activation of CYP2C9 activity by dapsone.
Dr. Hutzler continues to be interested in molecular mechanisms of cytochrome P450 metabolism and atypical kinetics. He has accepted a postdoctoral position at Pharmacia Corporation in Kalamazoo, Michigan, where he will be working with Dr. Larry C. Wienkers studying P450 enzyme-substrate and enzyme-effector interactions at the molecular level to help provide a better understanding of observed enzyme kinetic profiles. While at West Virginia University, Dr. Hutzler was elected a member of the Rho Chi honor society for pharmacy students and has been an AFPE (American Foundation for Pharmaceutical Education) fellow for the past 3 years.
 Timothy Tracy received a Bachelors of Science degree in pharmacy from Ohio Northern University (Ada, OH) in 1983. He then practiced community and hospital pharmacy for 2 years before entering graduate school. In 1988, he received the Ph.D. degree in pharmacy from Purdue University (West Lafayette, IN) under the direction of Dr. Curtis Black. His doctoral work involved the study of the pharmacokinetics and pharmacodynamics of calcium channel blockers as agents to treat preterm labor.
Timothy Tracy received a Bachelors of Science degree in pharmacy from Ohio Northern University (Ada, OH) in 1983. He then practiced community and hospital pharmacy for 2 years before entering graduate school. In 1988, he received the Ph.D. degree in pharmacy from Purdue University (West Lafayette, IN) under the direction of Dr. Curtis Black. His doctoral work involved the study of the pharmacokinetics and pharmacodynamics of calcium channel blockers as agents to treat preterm labor.
Dr. Tracy then began work as a postdoctoral fellow in clinical pharmacology at the Indiana University School of Medicine (Indianapolis, IN) under the direction of Dr. Steven Hall and Dr. Craig Brater. During this time, his primary project involved the study of the chiral inversion of ibuprofen, including formation, epimerization, and hydrolysis of the ibuprofenyl-CoA intermediates. In 1992, he joined the faculty of the West Virginia University School of Pharmacy, where he currently is an Associate Professor of Clinical Pharmacology. His current research interests involve substrate specificity and regulation of CYP2C9 and mechanisms involved in atypical kinetic profiles of P450-mediated reactions.
Footnotes
-
This work was supported in part by Public Health Service Grant GM-63215 (T.S.T.). J.M.H. was supported in part by a fellowship from the American Foundation for Pharmaceutical Education.
- Abbreviations used are::
- P450
- cytochrome P450
- LAAM
- levo-α-acetylmethadol
- b5
- cytochrome b5
- The American Society for Pharmacology and Experimental Therapeutics